L. I. Nadaf*1, K. S. Venkatesh2
1Department of Chemistry SECAB’s ARS Inamdar Arts,Science and Commerce College Bijapur- 586109 Karnataka, India.
2Department of Materials Science,Gulbarga University,Gulbarga-585106, Karnataka, India.
Corresponding Author Email: linadaf@rediffmail.com
DOI : http://dx.doi.org/10.13005/msri/120204
Article Publishing History
Article Received on : 08 Aug 2015
Article Accepted on : 10 Sep 2015
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
The polyaniline can be synthesized by oxidative polymerization of aniline. The PANI-based nano-composites can be synthesized by the method of polymerization. The oxides of copper are intrinsic p-type semiconductors with relatively small band gaps and show many attractive properties that can be utilized in a diversity of applications This paper includes synthesis of CuO nano-particles, and the polyaniline-CuO nano-composites with five different percentages of CuO nano particles. These samples were characterized by XRD and SEM.
KEYWORDS:
Polyaniline; CuO nano-materials; PANI-CuO nanocomposites
Copy the following to cite this article:
Nadaf L. I, Venkatesh K. S. Polyaniline-Copper Oxide Nano-Composites: Synthesis and Characterization. Mat.Sci.Res.India;12(2)
|
Copy the following to cite this URL:
Nadaf L. I, Venkatesh K. S. Polyaniline-Copper Oxide Nano-Composites: Synthesis and Characterization. Mat.Sci.Res.India;12(2). Available from: http://www.materialsciencejournal.org/?p=3689
|
Introduction
Materials have been very important in the history of human endeavors. One of the more important groups of materials in our lives today is composite material. The man made composite material, is a three-dimensional combination of at least two chemically distinct materials, with a distinct interface separating the components, created to obtain properties that cannot be achieved by any of the components acting alone1. Nanocomposites are composites in which at least one of the phases shows dimensions in the nanometer range2. These materials have been emerged as suitable alternatives to overcome limitations of micro composites and monolithic. The polymers having poly-conjugated structures and possess poor electrical conductivity but the oxidized polymers exhibit appreciable electrical conductivity3-6. Among conjugated polymers Polyaniline (PANI) is unique among the conducting and the most researched organic conducting polymer which is easy to synthesize, having fair good chemical stability and widely studied for electronic and optical applications. A number of metal and metal oxide particles have been encapsulated into the conductive polymer to form nano-composites. In the last 20 years, there has been a strong emphasis on the development of the PANI-based nanocomposites. These nano-composites exhibit the electrolytic and gas sensing properties7.
The oxides of copper are intrinsic p-type semiconductors with relatively small band gaps and show many attractive properties that can be utilized in a diversity of applications8.The PANI-CuO nano-composites exhibit unexpected hybrid properties synergistically derived from both components. In this paper, we describe the synthesis of PANI and CuO nano-composite materials by polymerization method. The prepared samples were characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM)9.
Materials and Methods
The chemicals used such as copper (II) nitrate, aniline, ammonium persulfate, ammonia solution, acetone, ethanol, and concentrated HCl were of analytical grade. The double distilled was used for the experimental work.
Synthesis of Polyaniline
Here we adopted oxidative polymerization of aniline. The solution of 0.5 M aniline and 0.5M ammonium persulphate was prepared in 0.5M HCl in two separate flasks. These two were mixed slowly under magnetic stirring, the colourless solution slowly turned to green. It was left for few hours without stirring to settle the powder, and then it was filtered using Bysner funnel. The dark green coloured residue in the form of paste was obtained. Finally the paste was washed with acetone to remove short chain molecules of aniline that were soluble in acetone and the paste was allowed to dry completely. The dried material was grinded to fine powder using Passel motor and the powder is known as the conducting PANI and it was kept sealed for further process.
Synthesis of CuO nano particles
The 6g of copper turnings were collected in a 100ml beaker and the 24ml of HNO3 is slowly added. The copper turnings were oxidised, solution was highly concentrated greenish colour to brownish-green colour. This concentrated solution appears bluish green, when it was diluted with 600ml distilled water, and the nitrate ions were displaced in the coordinate sites around the copper ions by the molecules of water, the solution colour was changed blue. The 10% sodium chloride was drop wise added to the solution and stirred. The obtained copper hydroxide precipitate was filtered, and then washed with distilled water several times and dried at room temperature. The so obtained material was calcinated at 400oC for 2hours; the resultant black coloured sample was collected for further process.
Synthesis of PANI-CuO nano composites
The five different nano-composite samples of PANI-CuO were prepared by following method: The known weight of CuO was added to the mixture of aniline and the hydrochloric acid and stirred for one hour. Then ammonium persulfate was added to the prepared mixture and stirred at low temperature for six hours. The solution was then filtered and the green paste was first washed with acetone and then by distilled water. The paste was then dried in air for two days and the in oven at 70oC for eight hours. The resultant sample was dark green powder, collected in a bottle for further process. The prepared sample was estimated as 10wt% CuO added during the polymerization of aniline. The procedure was repeated for the preparation of 15wt%, 20wt%,25wt% and 30wt% PANI-CuO nano composites.
Results and Discussion
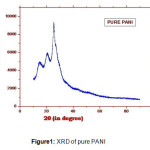
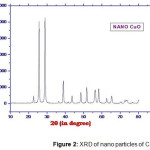
The XRD patterns of pure PANI and nano CuO are shown Figure-1 and 2 respectively. The XRD pattern of pure PANI shows three broad peaks at 2θ values 15o, 20o and 25o and indicates its amorphous nature. The Figure-2, reveals the crystalline structure of the sample, the peaks are observed at 2θ values 25.76o , 29o ,and hence the CuO nanoparticles posses the monoclinic structure. The average crystallite size of the CuO nano crystalline is estimated about 20 nm by Scherrer’s formula.
Figure 1: XRD of pure PANI
Figure 2: XRD of nano particles of CuO
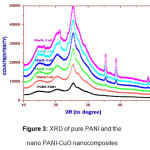
Figure 3: XRD of pure PANI and the nano PANI-CuO nanocomposites
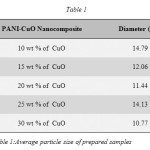
The Figure-3 shows the XRD patterns of prepared PANI-CuO nanocomposites with different weight percentages (10 %, 15 %,20 %,25 % &30 %) of CuO. The common peak at 2θ=25o, is present in all five prepared composites, indicates that the PANI retains its identity in composite phase. The heights of peaks at 2θ=35.42o, 2θ=38.75o and 2θ= 48.62o increases with increase in the weight percentage of CuO nanoparticles. Thus the nanocmposite gradually take the transition from the amorphous to crystalline phase with increase in the percentage of the CuO nanoparticles. Average crystallite size of the nanocomposites was calculated using Scherer formula and presented in Table-1.
Table 1: Average particle size of prepared samples
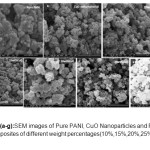
The SEM images of pure PANI, CuO nanoparticle and the five nanacompistes are given in figures-4(a-g). Figure-4(a) shows that Scanning Electronic Micrograph (SEM) image of pure polyaniline, has amorphous nature and grains are well interconnected. The Figure-4(b) is the SEM image of CuO nanoparticles which shows the homogeneous distribution of spherical particles. The Figure-4(c) is the SEM image of 10 wt % of PANI-CuO composite here the grains are irregular in shape, the CuO oxide partilcles are not well dispersed.The Figures 4(d), (e), (f) and (g) are the respective SEM images of 15 wt % , 20 wt % , 25 wt % and 30 wt % PANI-CuO composites, with increase in wt% of CuO results increases the crystallinity of composite materials.
Figure 4 (a-g): SEM images of Pure PANI, CuO Nanoparticles and PANI-CuO nanocomposites of different weight percentages(10%,15%,20%,25%, & 30% of CuO)
Conclusion
PANI-CuO nano composites had been synthesised. The XRD pattern shows that the crystallization process changes with different weight percentage CuO. The degree of crystallinity increased in PANI-CuO nanocomposites with increase in weight percentage of CuO nanoparticles and clearly indicated the homogeneous distributions of nanoparticles in the polymer matrix. Morphology analysis of all the samples was done with help of SEM images.
Acknowledgement
This work was supported by research center, Department of materials science, Gulbarga University Gulbarga (Karnataka –India).
References
- Stejskal .J and Gilbert R. G. Pure and Applied Chemistry.74, 857-867 (2002).
CrossRef
- Roy R, Roy RA, Roy DM. 1. Materials Letters.; 4(8-9):323-328(1986).
CrossRef
- Farah Kanwal, Saira Ishaq, Tahir Jamil, J.Chem.Soc.Pak.31(6), 907-910(2002) .
- Schmidt D, Shah D, Giannelis EP. 2. Current Opinion in Solid State & Materials Science.; 6(3),205-212 (2002).
CrossRef
- Gleiter H. Nanostructured Materials. 1(1):1-19(1992).
CrossRef
- Gangopadhyay R, Amitabha D. Chemistry of Materials.; 12(7): 608-622(2000).
CrossRef
- Wang. C, Daimon .H., Onodera. T., Koda .T., Sun .S., Angewandte Chemie International Edition, 47, 3588-3591(2008).
- Mittiga.A., Salza.E.,Sarto.F.,Tucci.M. Vasanthi.R, Appl Phys Lett, 88 163502(2006).
- Raja .S., Deepa M., Indian Journal of Advances in Chemical Science 3(2) 198-203(2015).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal