Nagaveni Thallapalli1*, K. Kishore Kumar2 and C. S. P. Rao2
1Dept. of Mechanical Engineering, National Institute of Technology, Warangal, Telangana, India, Pin:506 004 and Dept. of Mechanical Engineering, University College of Engineering, Osmania University, Hyderabad, Telangana State, India, Pin:500 007
2Dept. of Mechanical Engineering, National Institute of Technology, Warangal, Telangana, India-506004
Corresponding Author Email: tnagaveni@gmail.com
DOI : http://dx.doi.org/10.13005/msri/130105
Article Publishing History
Article Received on : 25 Jan 2016
Article Accepted on : 22 Feb 2016
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this work, a novel method for the preparation of colloids has been studied for the fabrication of silicon nitride –Boron nitride composites. In the present work, the dispersion of mixed silicon nitride –Boron nitride powders in aqueous media was studied with the changes dispersant concentration, solution pH etc. Polyethylenimine (PEI) additive as a dispersant were used for Si3N4 and BN powders in aqueous media. Well-dispersed Si3N4 and BN powders in aqueous media were attained atthe 1 wt% PEI and pH 9. 40 vol% covered Si3N4/BN slurries with varying BN content was adapted for gel casting. The gel casted material waspreheated at normal room temperature, debindered at 6000C and sintered at 17000C. The sintered composite material composed mainly of alpha-Si3N4, beta-Si3N4, and h-BN. The prepared composite material shows uniform microstructure with faceted particles, α-Si3N4 and abundant pores.
KEYWORDS:
Dielectric properties; Gel-casting; Mechanical properties; Si3N4/SiO2 ceramic composites; Thermalproperties
Copy the following to cite this article:
Thallapalli N, Kumar K. K , Rao C. S. P. Preparation and Characterization of Si3N4-BN Ceramic Composites by Gelcasting. Mat.Sci.Res.India;13(1)
|
Copy the following to cite this URL:
Thallapalli N, Kumar K. K, Rao C. S. P. Preparation and Characterization of Si3N4-BN Ceramic Composites by Gelcasting. Mat.Sci.Res.India;13(1). Available from: http://www.materialsciencejournal.org/?p=3784
|
Introduction
Ceramic gelcasting was developed rapidly over the past decade due to its capability of nearer-net-configuration of fine ceramic segments. The main advantages of the gelcasting technique include more accuracy in its dimensional states and capability to produce complex shapes, as well as decreasing the manufacturing cost. In-situ polymerization of monomer solution in the presence of ceramic powder into a mold, able to crosslink the particles in a gelled part, then obtained ceramic composites dried at room temperature and sintered further.1-3 Gel casting was near net shaping method for ceramics to overcome some of the problems in the existing processing routes, mainlymoulding by injection method (e.g., long debinding time and/or flaw occurrence at the time of thermolysis of binder) and slip casting method (e.g., slow rate of casting, low strength for green machining). The proposed gel casting technique for fabricating high-quality, complex-shaped ceramic structure is a promising technique for the production of ceramics at a less cost than conventional fabrication techniques. Moreover, gelcasting technique had number of applications from the range of accelerator magnets to artificial bone. The main advantage of gelcasting is able to form near-net-shapes, adequate green strength, shorter molding time, and low or absence of organic contaminants in the dried ceramics. Therefore, gel casting technique was broadly applied to prepare variety of ceramic materials.4
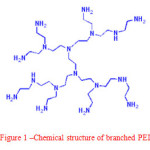
In gelcasting process, ceramic suspension with high solid loading and also low viscosity is one of the most desirable key issues. To attain such type of ceramic suspension entity, dispersant selection and dispersant controlling, concentration of dispersant and pH must be consider. Among various polymers based dispersants, the chemical structure of branched polyethylenimine (PEI) as shown in Fig. 1, shows good nature of steric stabilization. Moreover, it contains high positive charge density that may allow it to adsorb negatively charged substrates, leads to produce the higher electrostatic forces in the suspension. Therefore, dispersion of ceramics in aqueous suspensions has been widely carried out by using strong cationic branched polymers like PEI.5
Figure 1: Chemical structure of branched PEI
Many studies reported the colloidal synthesis of Si3N4 in the previous literatures[6-7] but studies on colloidal synthesis of Si3N4/BN composites not been reported much in the literature.This work reports the preparation and optimization of Si3N4–BN aqueous suspension with the loading of high solid content and less viscosity. This work also reports the effect of various parameters such as concentration of PEI, pH of the solution on dispersion of Si3N4 and BN in aqueous media. Si3N4–BN rheological behavior in aqueous suspensions was investigated in detail in the present work. The objective of the work is to investigate the effect of BN content on the properties of the composites. The primary reason for using BN constant is to improve dielectric properties.
Materials and Method:
The commercially available key raw materials such as Si3N4 powders, h-BN powders, Ai2o3 and y2o3 () were obtained from SN E-1Ube industries Ltd, Japan. The purity of Si3N4 and BN powders were of99%. 0.5 mm median volume diameter (D50) of Si3N4 and BN powder was procured from the manufacturers. Polyethyleneimine manufactured by PEI, Aladdin Co. Ltd., Shanghai, China, had an average molecular weight of 10,000 were used as dispersant for the preparation of aqueous suspension.
A mixture of silicon nitride and BN powders was selected as starting materials. 2wt.% Al2O3 and 3 wt% y2o3 was also used as sintering aid which causes a better sintering of the ceramic coating through next step of preparation. Zeta potential of Si3N4 and BN in PEI dispersant were measured as a function of pH. To produce slurries, powders were mixed with various amounts of PEI (0.4–1.5wt.%) as a dispersant. The pH of slurries was also set 10. To investigate the effect of pH on Si3N4–BN suspension properties, slurries with were prepared.. Hydrochloric acid and Ammonium hydroxide solutions were used to adjust pH value.
The materials which are used for gelcasting process was shown in Table 1.The Material Codearelisted in Table2, S0S5S10, and S15denote the SiO2 is added0vol. %, 5vol.%, 10vol.% and 15vol%. Table 1 shows the composition of slurries. Premix solution was prepared by mixing Methacrylamide (MAM) and N,N’-methylenebisacrylamide (MBAM) in distilled water with a 1 wt% of PEI. The premixed aqueous solution contained 10 wt.% monomer content, and 5:1 ratio of MAM to MBAM. Ceramic powders were added to premix solution maintaining the loading of solids in the suspensions as 50 vol%. Suspensions were grinded using ball- mil over a period of 24 h in a plastic bottle to cut down the agglomerates and to attain the uniformity in size.Hydrochloric and ammonia solution was used to adjust the pH value to 10.5.The final obtained suspension was degassed in 5-10 min then 10 wt% aqueous solution of ammonium persulfate, APS initiator and catalyst (tetramethylethylenediamine, TEMED) were introduced. Afterward, the ceramic suspension is used cast into the nonporous mold and dried under controlled humidity and binder was burned out at 5500C for 2 h, with the increment of 20 C/min. Then the samples have been sintered at the temperature of 1700 0C for 2h in N2 atmosphere, followed by natural cooling.
Table 1: Raw materials used in the experiments
|
Materials
|
Function
|
Supplier
|
|
Silicon nitride (Si3N4)
|
Starting Powder
|
Denka Kogaku, Japan, d50~ 4.2 µm
|
|
h- Boron Nitride (BN)
|
Starting Powder
|
Sigma Aldrich, 99.5% Purity,particlesizebetween 1-5µm
|
|
Yttriumoxide (Y2O3)
|
Sintering aid
|
Alfa aesar,USA, averageparticlesizearound 50nm
|
|
Alumina (Al2O3)
|
Sintering aid
|
Alfa aesar,USA, averageparticlesizearound 100nm
|
|
Methabisacrylamide (MAM)
|
Monomer
|
MAM, Sigma Chem. Co., USA
|
|
N,N’-Methylenebisacrylamide (MBAM)
|
Crosslinker
|
MBAM, Sigma Chem. Co., USA
|
|
Polyethyleneimine (PEI)
|
Dispersant
|
Zschimmer & Schwarz GmbH
|
|
Distilled Water
|
Solvent
|
|
|
Ammonium persulphate (APS)
|
Initiator
|
APS, Sigma Aldrich, USA
|
|
N,N,N’,N’-Tetramethylethylenediamine (TEMED
|
Catalyst
|
Sigma Aldrich, USA
|
Materials Characterization
Zeta potential measurements were carried out as a function of pH of the very dilute suspensions (0.05 vol%) using Zeta potential analyzer (Zetasizer Nano ZS, Malvern, UK). Rheological characterization was performed on rheometer (Physica MCR 51,Anton PaarGmbH) at 250C with cone and plate attachment. A massive density of green and sintered materials was analyzed by Archimedes’ principle. X-ray diffraction (XRD; Rigaku, D/max2550HB +/PC) were used to identify the phase composition of sintered samples. Scanning Electron Microscopy (SEM; JEOL, JSM-6460LV) has been used to characterize the microstructure of sintered samples. Mechanical properties of the ceramics, were characterized by the three-point bending strength method at normal room temperature was carried out according to the ASTM D-143 (1996) standards on specimen bars with dimensions of 40mm×4mm×3 mm at a crosshead speed of 0.5 mm/min on electronic Universal Testing Machine(Dak Systems, India).The fracture toughness (KIC) of the specimen dimension of 2.5 mm × 5 mm × 30 mm and notch length of 2.5 mm were used for the investigation by single edge notched beam (SENB) method.
Results and Discussion
Effect of pH on Zeta potential of suspensions
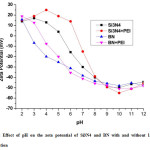
Electrostatic stabilizationis widely used to stabilize the ceramic suspensions by the repulsive forces of net surface charges between the particles. Fig. 2 shows the Zeta potential of Si3N4 or BN powders as a function of pH. The isoelectric point(iep) for Si3N4 and BN powder with PEI locates at the pH value of 6.5 and 3.7 respectively. Increasing of pH value from 2 to 12, Zeta potential was also increased negatively up to -55.3mV for Si3N4 and -51.2mV for BN powders, respectively. Addition of 1wt% PEI intothe suspensions,made the pHiep of both the powders transferred to basic region and Zeta potential attains more negative value, especially whenever the suspensions pH>7.It was observed that the changes in Zeta potential value and pHiep of each particles of powder is mainly caused by the addition of dispersant, creates the positive charge on the surfaces or maximize the negative charges on the surfaces of the particles.8-9 The cationic PEI has higher affinity to the negatively charged surface hence the stronger effect of the dispersant on the particle surface charge was observed in lower pH region.
Figure 2: Effect of pH on the zeta potential of Si3N4 and BN with and without 1wt% PEI concentration
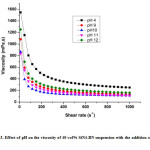
Figure 3: Effect of pH on the viscosity of 40 vol% SiN4-BN suspension with the addition of 1 wt% PEI
For making the Si3N4– BN composite, the following operational parameters such asrheological properties, pH, solid loading as well as low viscosity of the suspension is very important. In order to find the optimum pH value ofstable suspensions, the pH of the suspensions were readjusted to observe the viscosities of the Si3N4– BN suspensions containing 40vol% of solids loading, as shown in Fig. 3.The viscosity of the suspension decreases whenever the pH increase from 5 to 10, and also observed that viscosity slightly increase wheneverpH increases from 10 to12[10]. It was observed that lower viscosity were attained at pH=10. These results are agreed well with the results of zeta potential analysis, it was conformed that optimum pH of Si3N4– BN aqueous suspension was 10.
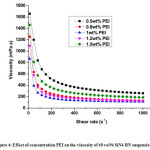
Figure 4: Effect of concentration PEI on the viscosity of 40 vol% SiN4-BN suspension..
As discussed earlier, the zeta potentials of Si3N4 and BN particles shown stronger inter- particle forces exhibits stronger repulsions at lower and higher pH values. Hence, the variations in zeta potentials able to affects the rheological behaviour of Si3N4– BN suspensions. Fig. 4 exhibits the viscosities of Si3N4– BN suspension as a function of shear rate with various concentrations of PEI. The rheological behavior of the Si3N4– BN aqueous suspensions is completely dependent on the quantity of PEI added. The addition of 1 wt% PEI to the suspension was shown the lowest viscosity. 1 wt% of PEI has been found as the optimum concentration to disperse the Si3N4– BN powder mixture in aqueous media. It was also shown the less viscosity value (as shown in Fig. 4) and high zeta potentials. For every group, the flow curve deviates from a Newtonian behavior, its viscosity becomes lower as the shear rate is increased, which is known as shear-thinning in rheology.11 Besides, it was observed that the viscosity of slurry reduces with increase of dispersant dosage and an optimum value of the dispersant dosage was found.
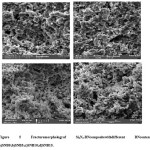
Figure 5: Fracture morphology of Si3N4-BN composite with different BN content. a) SNB0;b)SNB5;c)SNB10;d)SNB15.
Si3N4-BN composites samples having fracture surfaces were observed by SEM analysis. From Fig.5,one can observe thatBN and Si3N4 are homogenously distributed in the pores of the composite. The pores are distributed evenly and it was found that the average diameter of the pore is around 4μm. It was found that the microstructures are homogeneous, and the small amount of β-Si3N4 could be identified in the SEM micrograph due to the elongated grains. From Figure,it can be seen that the amount of long-bar-shapedβ-Si3N4 reduces while increase of BN content.
From the microstructure analysis, it could be observed that the samples composed of bonded particles and very less columnar grains with more aspect ratio, exhibiting few α/β phase transitions, and XRD analysis are well consistent with these results. It was also observed that the pores distributed homogeneously in the ceramic matrix, and the connected open pores are generated by stacking Si3N4 particles.
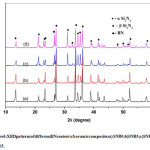
Figure 6: XRD patterns of different BN content of ceramic composites a) SNB0;b)SNB5;c)SNB10; d) SNB15.
Fig. 6 shows the phase compositions of porous Si3N4-BN ceramics composed of different contents of BN quantity. The XRD Pattern shown in figure indicates that the main crystal phase of the composites is β-Si3N4, α-Si3N4 and h-BN. The diffraction intensities of α-Si3N4 increases and the β-Si3N4 decreases with the increase of h-BN content.Transformation of alpha to beta phase in Si3N4 was achieved, but the transformation to β-Si3N4 was hinders as the BN content increases.
Conclusions
Gel casting method was successfully applied for the fabrication of Si3N4-BN ceramic composites and pressure-lesssintering.The effects of concentration of the PEI and pH on zeta potentials of Si3N4–BN powders were investigated in detail. The effect of BN content on microstructure of the Si3N4-BN ceramic composites was studied. Zeta potential values for both powders obtained as negative whenever the pH increases from 2 to 12 or with the addition of 1wt% PEI. Well- dispersed Si3N4 suspension and BN suspension were obtained by the addition of 1 wt% of PEIat pH 10. The rheological behavior for the Si3N4–10BN suspensions was also studied and discussed well. The uniform microstructures of the samples withuniform pore size distribution attained with gel casting technique. From the microstructure it was shown that the amount of long-bar-shapedβ-Si3N4 decreases while increase of BN content.
References
- Young A. C., Omatete O. O., Janney M. A and Menchhofer P. A. Gelcasting of alumina. Journal of the American Ceramic Society. 1991;74:612-618.
CrossRef
- Omatete O. O and Nick J. J. Improved Gelcasting Systems. In: 23rd Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: A: Ceramic Engineering and Science Proceedings: John Wiley & Sons, Inc. 2008;241-248.
- Omatete O. O., Janney M. A and Nunn S. D. Gelcasting: From laboratory development toward industrial production. Journal of the European Ceramic Society. 1997;17:407-413.
CrossRef
- Omatete O. O., Bleier A., Westmorel C. G and Young A. C. Gelcast Zirconia-Alumina Composites. In: Proceedings of the 15th Annual Conference on Composites and Advanced Ceramic Materials: Ceramic Engineering and Science Proceedings: John Wiley & Sons, Inc. 2008;2084-2094.
- He R., Hu P., Zhang X., Han W., Wei C and Hou Y. Preparation of high solid loading, low viscosity ZrB2–SiC aqueous suspensions using PEI as dispersant. Ceramics International. 2013;39:2267-2274.
CrossRef
- Dai J. Q., Huang Y., Xie Z. P., Xu X. L and Yang J. L. Effect of Acid Cleaning and Calcination on Rheological Properties of Concentrated Aqueous Suspensions of Silicon Nitride Powder. Journal of the American Ceramic Society. 2002;85:293-298.
CrossRef
- Jia D., Shao Y., Liu B and Zhou Y. Characterization of porous silicon nitride/silicon oxynitride composite ceramics produced by sol infiltration. Materials Chemistry and Physics. 2010;124:97-101.
CrossRef
- Varga I., Csempesz F and Záray G. Effect of pH of aqueous ceramic suspensions on colloidal stability and precision of analytical measurements using slurry nebulization inductively coupled plasma atomic emission spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy. 1996;51:253-259.
CrossRef
- Huang Y., Zhou L., Tang Q., Xie Z and Yang J. Water-Based Gelcasting of Surface-Coated Silicon Nitride Powder. Journal of the American Ceramic Society. 2001;84:701-707.
CrossRef
- Zhang Q and Gu M. Rheological properties and gelcasting of concentrated aqueous silicon suspension. Materials Science and Engineering: A. 2005;399:351-357.
CrossRef
- Liu Y., Gao L and Guo J. Comparative study on the stabilizing effect of 2-phosphonobutane-1,2,4-tricarboxylic acid and citric acid for alumina suspensions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2001;193:187-195.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal