Study of Proton Conducting PVdF based Plasticized Polymer Electrolytes Containing Ammonium Fluoride
R. Kumar1*, Shuchi Sharma1,2, N. Dhiman2 and D. Pathak2
1Department of Physics, Goswami Ganesh Dutt Sanatan Dharam College, Hariana, Hoshiarpur, Punjab – 144 208, India
2Department of Physics, Sri Sai University, Palampur, Himachal Pradesh – 176 081, India.
Corresponding Author Email: rajiv_parashar17@yahoo.co.in
DOI : http://dx.doi.org/10.13005/msri/130104
Article Publishing History
Article Received on : 18 Mar 2016
Article Accepted on : 05 Apr 2016
Article Published : 05 Apr 2016
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Polymer electrolytes based on polyvinyledene fluoride (PVdF) and ammonium fluoride (NH4F) have been prepared and characterized. Films of polyvinyledene fluoride and ammonium fluoride have been prepared by solution casting technique using tetrahydrofuran (THF) as a solvent. Maximum conductivity of 1.17 x 10-7 S/cm at room temperature has been obtained for polymer electrolytes containing 10wt% NH4F. The conductivity of polymer electrolyte has been increased by three orders of magnitude from 10-7 to 10-4 S/cm with the addition of dimethylformamide (DMF) as plasticizer. The increase in conductivity has been explained to be due to the dissociation of undissociated salt/ion aggregates present in the polymer electrolytes with the addition of high dielectric constant plasticizer (DMF). Maximum conductivity of 1.26 x 10-4 S/cm has been observed for plasticized polymer electrolytes. The variation of conductivity with temperature suggests that these polymer electrolytes are thermally stable and small change in conductivity with temperature is suitable for their use in practical applications like solid state batteries, fuel cells, electrochromic devices, supercapacitors etc.
KEYWORDS:
Conductivity; Tetrahydrofuran; Dimethylformamide; Plasticizer; Ammonium fluoride
Copy the following to cite this article:
Kumar R, Sharma S, Dhiman N, Pathak D. Study of Proton Conducting PVdF based Plasticized Polymer Electrolytes Containing Ammonium Fluoride. Mat.Sci.Res.India;13(1)
|
Copy the following to cite this URL:
Kumar R, Sharma S, Dhiman N, Pathak D. Study of Proton Conducting PVdF based Plasticized Polymer Electrolytes Containing Ammonium Fluoride. Mat.Sci.Res.India;13(1). Available from: http://www.materialsciencejournal.org/?p=3813
|
Introduction
Polymer electrolytes are important category of superionic conductors, which gained importance because of their applications in different solid state ionic devices. Research on polymer electrolytes started in early seventies, when complexation of alkali metal salts (MX) with high molecular weight polyethylene oxide (PEO) have been reported in 1973,1 but these materials became important only when their technological significance as electrolytes for solid state batteries was highlighted by Armand et al., in 1978.2 These materials are interesting due to their special properties like ease of preparation in different shapes and forms, wide range of composition and hence wider control of properties, good electrode-electrolyte contact etc.3-7 These electrolytes are extensively studied with various alkali metal salts, divalent metal salts and transition metal ions, silver salts etc. due to their potential use in various solid state ionic devices.8-10 Similarly proton conducting polymer electrolytes based on PEO and various ammonium salts have also been extensively studied due to their potential applications in batteries, sensors, electrochromic devices, fuel cells etc.11-17 However the complexation of polymer with fluoride salts has not been studied due to the insolubility of simple fluorides. Only a few reports on the polymer electrolytes based upon fluoride salts exist in the literature.14,15,18-19 As PEO is more crystalline in nature so their electrolytes posses low ionic conductivity at room temperature, which limits its use in practical applications. Various attempts have been made to increase the conductivity of these electrolytes. The conductivity of polymer electrolytes can be increased either by using of polymer which is amorphous or by additing plasticizer.20-22 The addition of plasticizer has been reported to result in an increase in amorphous content along with a decrease in the glass transition temperature.14-16, 23-26 Studies on the variation of conductivity of polymer electrolytes with salt concentration has been reported to show the presence of ion aggregates at higher salt concentrations, which do not take part in the conduction process.15,23,27-29 The dissociation of ion aggregates shall also result in an increase in the number of free ions, which results in an increase in conductivity. Generally solvents like PEG, PPG etc. are used as plasticizers which result in an increase in amorphous content along with a decrease in the glass transition temperature of the polymer electrolytes. As it has been observed that ion aggregates are present in polymer electrolytes at higher salt concentration are dissociated with the addition of high dielectric constant plasticizer and hence the conductivity increases.14-16, 30-34
Polyvinylidene fluoride (PVdF) which is an amorphous polymer with dielectric constant (Î = 8.4) has been used in the present paper. Films of PVdF and PVdF+NH4F for different concentrations of salt were prepared by solution casting technique using tetrahydofuran (THF) as solvent. Dimethylformamide (DMF) with high dielectric constant (Î = 36.7) has been used as a plasticizer. The variation of conductivity of polymer electrolytes as a function of salt concentration, plasticizer concentration and temperature has been studied.
Materials and Mathods
Polyvinylidene fluoride (PVdF) (Fluka), dimethylformamide (DMF) (Merck), ammonium fluoride (NH4F) (Reidel) and tetrahydrofuran (THF) (Merck) has been used as the starting materials. Polymer electrolytes in the film form were prepared by solution casting method with THF as the solvent. Ammonium fluoride in stoichiometric quantities was dissolved in THF and then PVdF was added to it and the mixture was stirred to obtain a homogenous solution. The homogenous solution thus obtained was then poured into polypropylene dishes and the solvent was allowed to evaporate and free standing polymer electrolyte films were obtained. The transparent films having thickness ~100 microns were used for various experimental studies. The conductivity was measured by complex impedance spectroscopy with HP4284A precision LCR meter operating in the 20Hz – 1MHz frequency range using a sample holder with silver/platinum electrodes.14-15,35-39
Results and Discussion
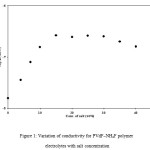
The variation of conductivity of PVdF+NH4F polymer electrolytes as a function of salt concentration is shown in Figure 1. From Figure 1, it has been observed that conductivity increases with an increase in salt concentration, reaches a saturation value and even a small decrease in conductivity is observed at higher salt concentrations. Maximum conductivity of 1.17×10-7 S/cm at room temperature has been obtained for polymer electrolytes containing 10wt% NH4F. At low salt concentrations, the conductivity increases linearly and salt is assumed to be completely dissociated and nearly all ions are available for conduction. With further increase in salt concentration, the conductivity shows a deviation from linear behavior, reaches a maximum value and then shows a small decrease with the addition of salt. The deviation in linearity of the plot at medium salt concentration values is generally explained to be due to the formation of ion aggregates, which do not take part in the conduction process. At higher salt concentrations, the formation of ion aggregates increases and conductivity attains a saturation value. But with the addition of salt, the viscosity of polymer electrolytes also increases due to complexation of salt with polymer, which reduces the mobility and hence conductivity decreases.14-17
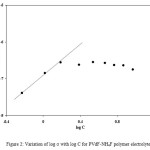
The presence of ion aggregates at higher salt concentrations can be checked on the basis of ‘mass action consideration’.40 According to this method, if σ is the conductivity of the polymer electrolyte and C is the concentration of salt, then a linear variation between log σ and log C shows that ion aggregates are not present and any deviation from linear variation suggests the presence of ion aggregates.41-45 Figure 2 shows the variation of log σ and log C for PVdF + xNH4F polymer electrolytes. In Figure 2, a linear variation is observed at low salt concentrations (0-10wt%), which suggests that ion aggregates are not present in this concentration range. But at higher salt concentration (> 10 wt%), a deviation from the linear behaviour is observed, which suggests that ion aggregate formation takes place and these results are in good agreement with the results of conductivity variation with salt concentration as given in Figure 1. The maximum conductivity observed for these polymer electrolytes is 1.17 ´ 10-7 S/cm, which is very small for their use in practical applications. The conductivity of these electrolytes should be increased by two to three orders of magnitude for their use in practical applications. It can be achieved either by using a polymer which is amorphous in nature or by adding a suitable plasticizer to the polymer electrolytes as the amorphous phase in polymer-salt type polymer electrolytes is the high conducting phase, so an increase in amorphous content of these polymer electrolytes shall result in an increase in conductivity. So, highly amorphous polymer (PVdF) and high dielectric constant plasticizer (DMF) (Î = 36.7) have been used in the present study.
Figure 1: Variation of conductivity for PVdF–NH4F polymer electrolytes with salt concentration
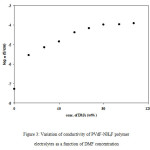
The effect of addition of DMF on the conductivity behaviour of PVdF + NH4F polymer electrolytes was studied for polymer electrolytes containing 30 wt% NH4F. This salt composition was in the high salt concentration region and was choosen due to presence of undissociated salt as well as ion aggregates. The conductivity of plasticized polymer electrolytes was measured as a function of DMF concentration (wt% of polymer) and the results obtained are shown in Figure 3. From Figure 3 it has been observed that with the addition of DMF the conductivity increases initially, then reaches a saturation value and even a small decrease is observed at still higher plasticizer concentrations. The conductivity of polymer electrolyte increases by nearly three orders of magnitude (i.e. from 10-7 to 10-4 S/cm) with the addition of DMF. Maximum conductivity of 1.26×10-4 S/cm at room temperature has been observed at 80wt% DMF. At low DMF concentration, the rise in conductivity is large which could be explained to be due to the dissociation of ion aggregates/undissociated salt present in the electrolyte in addition to an increase in the amorphous content. As the plasticizer content is further increased (upto 80wt%), the conductivity does not show an increase at the same rate and reaches a saturation value at higher plasticizer (DMF) concentrations. As the salt concentration in the electrolyte is kept constant (30 wt%), so initially undissociated salt/ion aggregates gets dissociated but with an increase in concentration of DMF, the amount of undissociated salt/ion aggregates decreases and hence the rate of increase in conductivity is not same and shows a saturation value at higher salt concentrations.
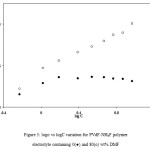
Figure 2: Variation of log σ with log C for PVdF-NH4F polymer electrolytes
Figure 3: Variation of conductivity of PVdF-NH4F polymer electrolytes as a function of DMF concentration
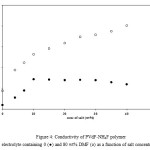
The role of DMF in the dissociation of undissociated salt and ion aggregates was also studied by adding fixed amount of DMF to the PVdF – NH4F polymer electrolytes containing different concentrations of salt. These electrolytes contain different concentrations of undissociated salt/ion aggregates. The conductivity of plasticized polymer electrolytes PVdF + xNH4F + DMF containing a constant concentration of DMF (equivalent to 80 wt% of polymer) as a function of salt concentration was measured and the results are given in Figure 4. For comparison the variation of conductivity of unplasticized polymer electrolytes PVdF+NH4F as a function of salt concentration is also included in Figure 4. From Figure 4, it has been observed that the conductivity of plasticized polymer electrolytes is higher than that of the unplasticized polymer electrolytes at all salt concentrations, but the increase in conductivity with DMF addition is different at different salt concentration. (i) At low salt concentrations (0 – 10 wt%) the increase in conductivity with DMF addition is small and is nearly one order of magnitude because the amount of undissociated salt shall be very small and ion aggregates may also not be present at such low salt concentrations as discussed above. The small increase in the conductivity with DMF addition may be due to an increase in amorphous content alongwith the dissociation of undissociated salt due to an increase in the overall dielectric constant of the electrolyte. (ii) At medium salt concentration (10 – 30 wt%), the increase in conductivity with DMF addition is by two orders of magnitude. In this concentration range, some undissociated salt and ion aggregates shall be present. The addition of plasticizer (DMF) increases the dielectric constant of the electrolyte, which results in the dissociation of ion aggregates and undissociated salt leading to an increase in the number of charge carriers (n) and hence conductivity increases. (iii) At high salt concentration (30 – 40 wt%), an increase of conductivity by three orders of magnitude has been observed with DMF addition. This could be explained to be due to the fact that more and more ion aggregates/undissociated salt shall be present and their dissociation with the addition of plasticizer leads to an increase in number of charge carriers and hence conductivity increases.
Figure 4: Conductivity of PVdF-NH4F polymer electrolyte containing 0 (●) and 80 wt% DMF (ᴏ) as a function of salt concentration
The role of DMF in the dissociation of ion aggregates and undissociated salt present in polymer electrolytes was also checked by “mass action consideration” as discussed in unplasticized polymer electrolytes.38 The variation of log σ and log C was studied for plasticized polymer electrolytes PVdF+xNH4F+DMF having different salt concentrations and constant plasticizer (DMF) content and the results are shown in Figure 5. For comparison the results of the variation of log σ vs. log C for unplasticized polymer electrolytes PVdF+NH4F are also included in Figure 5. In Figure 5, A linear straight-line behaviour has been observed at low salt concentrations for unplasticized PVdF+NH4F polymer electrolyte, which suggests the absence of ion aggregates at such low salt concentrations. But at higher salt concentrations, a deviation from the linear behaviour is observed, which suggests the presence of ion aggregates at higher salt concentration. For plasticized polymer electrolytes PVdF+xNH4F+DMF at different salt concentrations, the logσ vs. log C plot shows a linear behaviour at all salt concentrations (0–40wt. %) which suggests that ion aggregates are not present. Thus ion aggregates present in unplasticized polymer electrolytes get dissociated with the addition of DMF. The increase in conductivity with DMF addition is mainly due to the dissociation of ion aggregates/undissociated salt present in unplasticized polymer electrolytes which results in an increase in the number of free ions (n) and hence conductivity increases. If it would have been due to an increase in amorphous content alone, then the variation of log σ vs. log C for plasticized PVdF+NH4F polymer electrolytes would have been similar to that observed for unplasticized polymer electrolytes. But the behaviour observed in the present case is different. This supports the fact that the addition of plasticizer with dielectric constant more than the polymer helps in the dissociation of ion aggregates and undissociated salt45.
Figure 5: logσ vs logC variation for PVdF-NH4F polymer electrolyte containing 0(●) and 80(ᴏ) wt% DMF
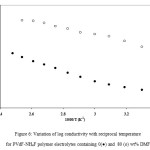
Conductivity of unplasticized and plasticized polymer electrolytes containing 30 wt% NH4F and plasticized with DMF (equivalent to 80 wt.% of polymer) was also measured as a function of temperature in the 20 – 120oC temperature range. The variation of log conductivity with reciprocal temperature is given in Figure 6. From Figure 6, It has been observed that the conductivity increases with an increase in temperature for unplasticized PVdF+30wt%NH4F as well as for plasticized PVdF+30wt%NH4F+80wt% DMF polymer electrolytes. Conductivity of plasticized polymer electrolytes is higher than that of corresponding unplasticized polymer electrolytes at all temperatures by two orders of magnitude. The change in conductivity with temperature is small over the whole temperature range (20-120oC) studied, which is desirable for their practical applications in devices like solid state batteries, fuel cells, electrochemical devices, supercapacitors etc.
Figure 6: Variation of log conductivity with reciprocal temperature for PVdF-NH4F polymer electrolytes containing 0(●) and 80 (ᴏ) wt% DMF
Conclusions
Maximum ionic conductivity of 1.17 x 10-7 S/cm at room temperature has been observed for unplasticized polymer electrolytes containing 10wt% NH4F. Salt is fully dissociated at low concentration and ion aggregates are present at higher concentrations of salt. The conductivity of unplasticized polymer electrolyte has been observed to increase by nearly three orders of magnitude from 10-7 – 10-4 S/cm with the addition of DMF as plasticizer. The large increase in conductivity has been explained to be due to the dissociation of undissociated salt/ion aggregates present in the polymer electrolytes with the addition of plasticizer (DMF). Maximum conductivity of 1.26 x 10-4 S/cm has been observed for plasticized polymer electrolytes PVdF+30wt%NH4F+80wt% DMF. The variation of conductivity with temperature shows a small change which is suitable for their use in practical applications.
Acknowledgements
The author (RK) is thankful to Department of Physics, Guru Nanak Dev University, Amritsar, Punjab and University Grants Commission, New Delhi for financial support in the form of Research Project No. 8-2(172)/2011 (MRP/NRCB).
References
- Fenton B. E., Parker J. M and Wright P. V. Polym. 1973;14:589-590.
CrossRef
- Armand M. B., Chabagno J. M and Duclot M. J. Extended Abstract on Second International Meeting on Solid Electrolytes, St. Andrews, Scotland. 1978;20-22.
- Feuillade G and Perche Ph. J. Appl. Electrochem. 1975;5:63-69.
CrossRef
- Scrosati B. In : Chowdari B. V. R., Yahaya M., Talib I. A and Saleh M. M. (eds). Solid State Ionic Materials, World Scientific, Singapore. 1994;47-50.
- Singh B., Kumar R and Sekhon S. S. Solid State Ionics. 2005;176:1577-1583.
CrossRef
- Kuila T., Achaya H., Srivastava S. K., Samantaray B. K and Kureti S. Mater. Sci. & Engg. B. 2007;137:217-224.
CrossRef
- Kamaluddin N., Latif F. A., Han C. C., Ibrahim R and Zamri S. F. M. Malays. J. Anal. Sci. 2015;19:658-662.
- Owens B. B and Argue G. R. Science. 1967;157:308-310.
CrossRef
- Chandra S., Superionic Solids – Principles and Applications. Amsterdam: North Holland. 1987.
- Gray F. M., Polymer Electrolytes. The Royal Society of Chemistry, Cambridge, U. K. 1997.
- McCallum J. R and Vincent C. A. Polymer Electrolyte Reviews-I & II, Elsevier Appl. Sci. Amsterdam. 1989
- Bohnke O. Proton Conductors: Solids, membranes and gels – materials and devices. In: Colomban P. (ed.). Cambridge Univ. Press. 1992;551-566.
- Shukla P. K and Agrawal S. L. Bull. Electrochem. 1996;12:32–737.
- Kumar M and Sekhon S. S. Euro. Polym. J. 2002;38:1297-1304.
CrossRef
- Sekhon S. S and Kumar M. Proceedings of 8th Asian Conference on Solid State Ionics. 2002;377-384.
CrossRef
- Sharma J. P., Yamada K and Sekhon S. S., Macromol. Symp. 2012;315:188–197.
CrossRef
- Kumar R. Inter. J. Sci. Technol. and Mgt. 2015;4:433-441.
- Takahashi T. In: Mahan G. D and Roth W. L. (eds.), Superionic conductors. Plenum Press, New York. 1976;379-394.
CrossRef
- Sharma S., Dhiman N., Pathak D and Kumar R. Ionics. 2016. DOI 10.1007/s11581-016-1721-2 in press
CrossRef
- Missan H. P. S., Chu P. P and Sekhon S. S. J. Power Sources. 2006;158:1472-1479.
CrossRef
- Noor M. M., Careem M. A., Majid S. R and Arof A. K. Mater. Res. Innov. 2011;15:157-160.
CrossRef
- Kumar R. Curr. Smart Mater. 2016, in press
- Michael M. S., Jacob M. M. E., Prabaharan S. R. S and Radhakrishna S. Solid State Ionics. 1997;98:167-174.
CrossRef
- Pradhan D. K., Samantaray B. K., Choudhary R. N. P and Thakur A. K. J. Power Sources. 2005;139:384-394.
CrossRef
- Bhide A and Hariharan K. Euro. Polym. J. 2007;43:4253-4270.
CrossRef
- Mustafa M. F., Ridwan N. I. M., Hatta F. F and Zahya M. Z. A. Malays. J. Anal. Sci. 2012;16:283-289.
- Watanabe M., Kanda M., Matsuda H., Tsunemi K., Mizoguchi K., Tsuchida E and Shinohara I. Macromol. Chem., Rapid Comm. 1981;27:41-744.
CrossRef
- Hashmi S. A., Kumar A., Maurya K. K and Chandra S. J. Phys. D: Appl. Phys. 1990;23:1307-1314.
CrossRef
- Sharma S., Dhiman N., Pathak D and Kumar R. i-Manager’s J. Mater. Sci. 2016,– in press
- Feuillade G and Perche Ph. J. Appl. Electrochem. 1975;5:63-69.
CrossRef
- Song J. Y., Wang Y. Y and Wan C. C. J. Power Sources. 1999;77:183-197.
CrossRef
- Ramesh S and Arof A. K. Mater. Sci. Engg. B. 2001;85:11-15.
CrossRef
- Rajendran S., Sivakumar M and Subadevi R. Mater. Letters. 2004;58:641-649.
CrossRef
- Kumar R and Sekhon S. S. J. Appl. Electrochem. 2009;39:439-445.
CrossRef
- Singh H. P., Kumar R and Sekhon S. S. Bull. Mater. Sci. 2005;28:467-472.
CrossRef
- Kumar R., Singh B and Sekhon S. S. J. Mater. Sci. 2005;40:1273-1275.
CrossRef
- Kumar R. i-Manager’s J. Mater. Sci., 2014;2:23-34.
- Kumar R. i-Manager’s J. Mater. Sci., 2014;2:12-17.
- Kumar R. Ind. J. Phys. 2015;89:241-248.
CrossRef
- Ratner M. A., In: MacCallum J. R and Vincent C. A. (eds), Polymer Electrolyte Reviews-I. Elsevier Applied Science, New York. 1987.
- Kumar R and Sekhon S. S. Ionics. 2004;10:436-442.
CrossRef
- Kumar R., Sharma J. P and Sekhon S. S. Euro. Polym. J. 2005;41:2718-2725.
CrossRef
- Kumar R and Sekhon S. S. Ionics. 2008;14:509-514.
CrossRef
- Kumar R and Sekhon S. S. Ionics. 2013;19:1627-1635.
CrossRef
- Kumar R. i-Manager’s J. Mater. Sci. 2015;3:21-27.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal