An Overview on Ruthenium Oxide Composites – Challenging Material for Energy Storage Applications
Dipanwita Majumdar*
Department of Chemistry, Chandernagore College, Hooghly, WB, India
Corresponding author Email: wbesdmajumdar@gmail.com
DOI : http://dx.doi.org/10.13005/msri/150104
Article Publishing History
Article Received on : 3 March 18
Article Accepted on : 28 March 18
Article Published : 30 Mar 2018
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Ruthenium oxides owing to their high specific capacitance have been widely identified as promising materials for electrochemical charge storage devices. However, high priced ruthenium precursors restrict their commercial usage. Accordingly, numerous explorations investigated the influences on capacitive behavior of ruthenium oxide on blending with varied materials like other metal oxides, activated carbons, conducting polymers, CNTs and functionalized graphene systems as composites. The aim had been to optimize the material cost without compromising with but improving the composite electrochemical performances. The scientific explorations reveal that the overall specific capacitance of composites is a strongly related to the ruthenium oxide (RuO2) present in the system since it is the main electro-active material providing the Faradaic pseudocapacitances besides the electrical double layer contributions from the base carbon component of the composite. Major progress in the theoretical and practical research and development in this particular field has enviced a large number of research articles and technical reports in the recent past. The current investigations focus on utilizing minimum amount of metal in the composite; upholding the synergistic effect from the metal oxide and the support (carbon materials generally) to obtain better electrochemical signatures. Optimization of important factors leading to reduced nanostructure agglomeration, minimum electrostatic resistance and ultrafast proton/electrons diffusion through the hollow porous structures may ultimately result to the theoretically expected specific capacitance. Nonetheless, to the best of knowledge of the author, there is no systematic review available pertaining to recent advancement of the composites of RuO2. Thus, this overview categorically narrates recent progresses on the fabrication, performances and achievements of ruthenium oxide composite as electrode material in energy storage applications which will be beneficial especially to the newcomers in this field of research.
KEYWORDS:
Electrode material; Nanocomposite; Ruthenium oxide; Supercapacitors; Specific capacitance
Copy the following to cite this article:
Majumdar D. An Overview on Ruthenium Oxide Composites – Challenging Material for Energy Storage Applications. Mat.Sci.Res.India;15(1)
|
Copy the following to cite this URL:
Majumdar D. An Overview on Ruthenium Oxide Composites – Challenging Material for Energy Storage Applications. Mat.Sci.Res.India;15(1). Available from: http://www.materialsciencejournal.org/?p=7093
|
Introduction
Electrostatic field storage has become one of the key issues of research today to cope up with high energy demands of the modern society. Recent advances in this technological field have lead to emergence of supercapacitors/ ultracapacitors or electrochemical capacitors. Supercapacitors (SCs) use electrodes with large specific surface area and thinner dielectrics to attain higher capacitances so as to have higher energy densities as well as power densities.1,2 Ultracapacitor is best suited for devices that demand high current at faster rate. Current researches focus on how to improve the energy density so as to be employed comprehensively in commercial domains. The performance of supercapacitor depends on several factors such as electrochemical characteristics of materials used as electrode; electrolyte added and voltage window.3,4 Nonetheless, most explorations are thrusted on the development and fabrication of new electrode materials that would capitulate better and till date improved performances.5,8
As previously stated, electrode materials play key roles in supercapacitor cell performances. Use of metal oxides or conducting polymers as electroactive materials has been found to provide significant increase in the specific capacitance and /or of the energy density. However, conducting polymers such as polypyrrole polyaniline, and polythiophene derivatives have limited practical-based applications, in spite of low production cost and ease of preparation, due to the fact that these organic based materials swell up and contract during charging or discharging respectively, degrade easily on long exposure to chemical environment and also the cycle life is considerably poor.9,12 Metal oxides owing to their thermal and mechanical stability are superior in that respect especially oxides, hydroxides of the transition metals such as RuO2, MnO2, VOx, WO3, Ni(OH)2, Co2O3,Co(OH)2, Fe3O4, etc., that have been widely tried as the electrode materials of pseudocapacitors.13,16
Among the wide range of metal oxides employed, hydrous ruthenium dioxide (denoted as RuO2.xH2O) has been considered to be the most important for practical implementations due to its superior reversibility, high-valued specific capacitance together with the very long cycle life.17,18 It is reported that Ruthenium oxide, RuO2, has outstanding electrocatalytic features for several electrochemical processes.19-20 Its’ important technological application lies in chlor-alkali industry, as the active component of dimensionally stable titanium anodes.21 The high specific capacitance is attributed to pseudocapacitance behavior (storage of Faradic charges) from the redox transitions of RuO2 involving the reversible mixed electron/proton exchange between the oxide active sites and the solution as shown below,22
RuO2 + YH+ + Ye− = RuO2−Y (OH)Y (0 ≤ Y ≤ 2).
The above electron-proton transfer reaction has been recently confirmed by means of electrochemical quartz crystal microbalance studies.22,23,24 Here, the diffusion of proton subdues hydrogen evolution in acid solution due to both proton intercalation as well as under-potential deposition on the RuO2−Y (OH)Y surface during redox switching thereby widening the range of operating voltage. This reaction has been theoretically estimated to provide an ultrahigh specific capacitance (~1400–2000Fg−1) of RuO2.25
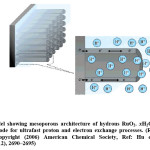
Amorphous structure is generally annealed at elevated temperatures under air to obtain the above properties.26 Unlike the anhydrous, crystalline Rutile (RuO2) phase which is a metallic conductor, the hydrous ruthenium oxide phase exhibits mixed proton/electron conductivity, that promotes it as a promising candidate for not only in supercapacitor devices (shown in Figure-1) but also for proficient electrocatalyst in direct methanol fuel cells (DMFCs).22,26 Based on the mechanism of simultaneous double injection and ejection of protons and electrons, pseudocapacitances of RuO2·xH2O have been optimized through balanced electronic and protonic transports in these systems.22,26 Therefore, effective control of electron and proton conducting pathways are the key issues for optimizing the pseudocapacitive performance of RuO2·xH2O.26-27
Figure 1: Model showing mesoporous architecture of hydrous RuO2. xH2O nanotubular arrayed electrode for ultrafast proton and electron exchange processes. (Reproduced on permission Copyright (2006) American Chemical Society, Ref: Hu et. al, Nano Lett., 2006, 6 (12), 2690–2695)
This mini-review is a representation of the recent development and advances achieved by this material regarding energy storage applications. It is noteworthy that few results are reported for these materials for practical implementations in spite of their superior features. Most results reported simply for a three electrode system and their performances differ significantly when employed in practical supercapacitor devices. Accordingly, it is very important to evaluate and analyze the obtained results for the better designing and development of active materials to meet the above requirements – an interesting and motivating subject for many scientists, especially to the new minds coming to this area of research.
Methods of Preparation
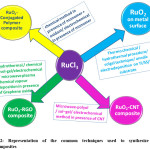
A variety of methods are available for the preparation of RuO2. xH2O material.27,30,31 Most commonly used wet chemical sol-gel method that employs RuCl2/ RuCl3 in presence/absence of surfactants and alkali followed by annealing at optimum temperatures to yield desired nanostructures.27 The material has primary particles sizes in the nano-range with a domination of orderly arranged nanopores of lower and higher size. Even electrochemical preparation involving direct cyclic voltammetric (CV) deposition on metal substrates such as titanium, steel, Si, Pt, Ni, etc. from an aqueous chloride solution are available in the literature.30,32 RuO2 can also be obtained by oxidation on Ru metal surface by cyclic voltammetry in H2SO4 electrolytes.31 Electrochemical deposition is a facile, one-step and cost-effective widely employed technique; where the texture, surface morphology and homogeneity of electrode can be tuned and controlled. During the cathodic deposition of RuO2, co-deposition of metal lowers the specific capacitance of the system. However, it can be overcome successfully by the anodic deposition of RuO2. Besides, atomic layer deposition (ALD) is also often employed to produce RuO2 films as well.33 Method of nanostructure preparation significantly changes the porosity of the material and hence the electrochemical performance also alters to appreciable extent. Figure-2 shows the outline for the formation of such RuO2 films as well as its composites using various methods such as sol-gel, hydrothermal, lithography, anodic electrodeposition, microwave plasma chemical vapor deposition, atomic layer deposition and various other methodologies to tune the electrochemical behavior in the resultant systems.
Results and Discussion
Various synthetic methodologies have been employed for preparing nanostructured hydrous RuO2 such as RuO2 nanoparticles, nanotubes, nanorod arrays, and hollow fusiform nanostructures to obtain better electrochemical performances.25,34,35 It has been observed that hollow RuO2 nanotubes structures show enormous advantages owing to their ability to provide ultrafast electrons and protons transfer, reduced ion-diffusion pathways, and high effective surface areas.36,37 However, the tedious fabrication process and the essential need for a binder to construct these electrodes highly influences the electrical conductivity and more often lead to significant lowering of charge storing performances.38 Thus to combat the existing problem, other alternative facile and effective binder-free route may be of great implication for enhancing the charge storage performances of hollow hydrous nano-RuO2-based supercapacitors. For instance, cathodic electrodeposition of RuO2 thin films onto Ti substrate showed specific capacitance of 788 Fg−1 for low RuO2 loading level (~ 1.4 mgcm−2).31 Even binder-free three-dimensional criss-crossed hollow hydrous ruthenium dioxide nanotubes fabricated on a Ti substrate electrode exhibited remarkable high-rate performance with a specific capacitance of 745 Fg-1 at a high current density of 32 Ag-1.39
However, to cope up with the high cost and environmental toxicity, reduced mass consumption of the Ru-precursor material has been opted without compromising with the electrochemical output by employing several mixed oxides, conducting polymer blends and obtained superior cyclic performances as shown in Table 1.
Table 1: Comparison of specific capacitance of RuO2 and its composites with metal oxide/conducting polymers
|
Table 1: Comparison of specific capacitance of RuO2 and its composites with metal oxide/conducting polymers
|
|
Materials
|
Special Capacitance (F·g−1) [Ref]
|
|
RuO2
|
745 [39]
|
|
NiO/RuO2
|
210 [40]
|
|
RuO2/Polyaniline
|
830 [41]
|
|
SnO2/RuO2
|
150 [42]
|
|
RuO2/TiO2
|
1263 [43]
|
|
RuO2/Ag2O
|
175 [44]
|
|
Cone-Shaped, well-aligned
Polypyrrole /RuO2 Nano-composite
|
302 F g-1 [45]
|
Even nanoporous gold have been used to improve the performance of ruthenium dioxide that exhibited the specific capacitance a value around 1500 F/g.46 Nonetheless, rapid agglomeration and easy detachment from the electrode surface promptly lowers the cyclic performances. Moreover, the effective production cost of the electrode using pristine RuO2 is large that limits its commercial usage considerably. Consequently, electrodes of ruthenium dioxide in various composite forms have been investigated for supercapacitor applications.
Various carbon forms for instances- Activated carbons (AC), carbon nanotubes (CNTs), carbon fibers(CFs)] and carbon aerogels (CAGs) are some of the materials that have been introduced in metal oxide composites for their improved conductivity and charge-storage capacities.47,50 Especially, the CNTs exhibit high mechanical strength and superior electrical conductivity that promote massive ion and electron transportation which is very much advantageous in high power electrochemical capacitors.51,52 Ruthenium oxide nanocomposites with carbon nanotubes, and carbon composites have been widely studied some of which have been summarized in Table-2.
Table 2: Elctrochemical performances of Ruthenium oxide –carbon based composites
|
TABLE-2: Elctrochemical performances of Ruthenium oxide –carbon based composites
|
|
Composite
|
Method of preparation
|
Performance
|
References
|
|
ruthenium oxide nanoparticles / multi-wall carbon nanotubes
|
microwave-polyol process
|
Specific capacitance of 450 Fg−1at scan rate of 10mVs−1 for potential window 0-1V, in 1 M H2SO4 electrolyte
|
[53]
|
|
ruthenium oxide/carbon nanocomposite
|
Sol-gel colloidal synthesis
|
Specific capacitance of 407 Fg−1 at scan rate of 1mVs-1 for potential window 0-0.9V, 1 M H2SO4 electrolyte.
|
[54]
|
|
Ruthenium oxide anchored multi-walled carbon nanotubes
|
Wet Chemical method
|
Specific capacitances of 213±16Fg−1 and 184±11Fg−1 obtained for Ru oxide/pure-MWCNT and Ru oxide/acid functionalized-MWCNT composites,1(M) H2SO4 scan rate of 20 mVs−1 for potential window 0-0.8V
|
[55]
|
|
RuO2 thin film deposited on
carbon paper
|
dip-coating method
|
Specific capacitance of 620 F g-1 1 (M) H2SO4 electrolyte, 0-0.8V potential scan rate of 5 mVs−1
|
[56]
|
|
ruthenium oxide particles anchored on activated carbon
|
sol–gel method
|
Specific capacitance of 208 mFcm-2 in electrolyte: 1-ethyl-3-methyl imidazolium tetrafluoroborate dissolved in acetonitrile at scan rate of 2 mVs−1for -1-0.5V potential range
|
[57]
|
|
hydrous RuO2/multi-walled carbon nanotubes composite
|
hydrothermal
|
Specific capacitance of 1585 F/g at scan rate of 2mV/s in the potential range 0–1.2 V.
|
[49]
|
|
ruthenium oxide/carbon nanotubes composite
|
deep-ultraviolet lithography
|
Specific capacitance of 208.5 mFcm−2 at scan rate 10 mVs−1 in a neutral Na2SO4 solution -0.3-0.7V potential window
|
[58]
|
Hu et al carried out a study on RuO2/CNT where they found a capacitance of 1340 F/g .59 Hydrous ruthenium oxide nano-composite with multi-walled carbon nanotubes (h-RuO2/MWCNT) were synthesized by facile hydrothermal method provided specific capacitance of 1585 F/g at a scan rate of 2 mV/s in the potential range of 0 – 1.2 V.49 RuOaHb/carbon black nanocomposite material prepared by impregnation method displayed impressive specific capacitance of about 700 Fg-1.60 RuO2 /MWCNT Electrode on Ti current collector prepared in two steps, firstly by growing MWCNT on Ti followed by electrodeposition of hydrous RuO2 showed superior specific capacitance of 1652 F/g at scan rate of 10 mV/s.61 These composites are characterized by superior electrochemical reversibility, high-energy density, excellent stability, and improved frequency response but limited by high production cost and tedious-fabrication methods. Moreover, typical CNTs varying in micrometers length simply gets aggregated into macroscopically entangled rope-bundles that drastically diminish their specific surface area and hence the overall charge storage capacity. Therefore, further innovative fabrication methods are essential to surmount the agglomeration problem as well as reduce the overall cost of the electrode material.
It is lately reported that CNTs, can be split into curved graphene nanosheets that on further oxidation by modified Hummers method.62 The so-obtained graphene oxide nanosheets possess unique hybrid characteristics of 1D nanotubes and 2D flat nanosheets which can be effectively employed in composite systems. The specific capacitances of the composite obtained by splitting MWCNTs with 40.0 wt% RuO2 loading by one-step hydrothermal synthesis without adding any reducing agent in 1 (M) KOH, 1 (M) H2SO4, and 1 (M) Na2SO4 are 453.7, 415.7, 287.5 Fg-1 respectively.62
Figure 2: Representation of the common techniques used to synthesize RuO2 nanocomposites
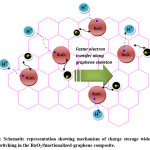
Among the various carbon substrates, graphene and its functionalized derivatives are suitable for electrochemical applications due to its tunable electrical conductivity, high surface area, and faster electron transfer rates besides low fabrication cost.63,64 Very recently, ruthenium dioxide/graphene sheet composite prepared by hydrothermal followed by low temperature annealing process showed specific capacitance of 551 Fg-1 at current density of 1.0 Ag-1 in 1(M) H2SO4 electrolyte solution with very low ruthenium content. The fine RuO2 particles with a size of 5–20 nm anchored on the graphene sheets experienced super electrochemical behavior (as shown in Figure-3,) leading to appreciable cyclic performances of 97.9 % capacitance retention after 1000 cycles.65 RuO2/reduced graphene oxide nano-ribbon composites containing 72.5 wt% RuO2 has been synthesized in wet chemical method in aqueous phase achieves specific capacitance up to 677 Fg-1 at the current density of 1 Ag-1 in three-electrode system using 1 (M) H2SO4 as electrolyte with excellent rate capability (91.8% retention at even 20 Ag-1) at high current density.66 Recent reports show that RuO2/graphene oxide composites when treated with polymeric dispersant Polyvinylpyrrolidone (PVP) faced less aggregation and exhibited significant cycling durability properties.67 Even ALD technique has been employed to abate RuO2 nanoparticles agglomeration, showing improved performances of ~92% capacitive retention after 4000 CV cycles at a scanning rate of 100mV/s.68 Vertically aligned nanocrystalline-RuO2 synthesized on Few Layered Graphene (FLG) nanoflakes, by microwave plasma chemical vapour deposition. The so-produced very small RuO2 nanoparticle (diameter <2 nm) containing composite exhibited high specific capacitance of ~ 650 F g-1 with good cyclic performances.69 RuO2/graphene sheet nanocomposites, with different amount of ruthenium loadings, prepared by combination of sol–gel and low-temperature annealing techniques furnished specific capacitance of 570 Fg−1 for 38.3 wt% Ru loading, with notable rate capability and enhanced electrochemical stability ( ~ 97.9% capacitive retention after 1000 cycles).70 In recent past, facile, scalable method of preparation of 3D – architecture with <5nm hydrous -RuO2 anchored onto graphene and CNT hybrid foam (RGM) have been reported showing impressive electrochemical stability with excellent capacitive performances.71
Figure 3: Schematic representation showing mechanism of charge storage wide electro-proton switching in the RuO2/functionalized-graphene composite
Conlcusion
The above studies reveal the following inferences about the RuO2 composites:
- High content or loading of RuO2 increases the specific capacitance due to its pseudocapacitive property.
- High conductivity compared of RuO2 to other metal oxides is an additional advantage for reversible electron transfer.
- RuO2 exhibits high cyclic electrochemical performances.
- High production cost of RuO2 limits its commercialization
- RuO2 /carbon composites (PPY, PANI, ACs, etc.) show high degree of agglomeration and poor electrochemical reversibility.
- RuO2 /CNTs although exhibit high specific capacitance but high rate of agglomeration, high production cost limits practical application.
- RuO2 /functionalized graphene systems till date exhibit moderate specific capacitance compared to RuO2 /CNTs but advantageous with respect to lower rate of agglomeration, lower production cost with improved cyclic performances.
Till date, appreciable capacitance values, good stability, lower production cost, along with reversible electrochemical behavior of the RuO2/functionalized graphene composites electrodes has high-ranked the system as very promising electrode material for future micro-supercapacitor devices. The composite has envisioned such superiority attributed to the synergistic contribution of individual constituents along with three dimensional structures that promote efficient and faster ion and electron transportation. Still it appears that to obtain better electrochemical behavior, optimization of all the components in the composites are very essential. Address to the problems related to high rate of electrode material agglomeration; minimization of production cost with controlled cyclic performances to achieve the desirable specific capacitance close to theoretical value for RuO2 based supercapacitors remains to be the main goal in the near future. Facile, green synthetic methods suitable for large-scale production with appropriate morphology ought to be a nonstop concern for the purpose of designing real supercapacitor devices.
Acknowledgement
DM acknowledges Chandernagore College, Hooghly, WB; Barasat Govt. College, Barasat, Kolkata; Department of Chemistry (Physical Chemistry Div.), Jadavpur University and Indian Association for the Cultivation of Science, Jadavpur, Kolkata, India for infrastructural facilities.
Funding Source
The author declares that the funding is done by author only.
Conflict of interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
- Burke A. Ultracapacitors: why how and where is the technology? J. Power Sources. 2000;91:37–50.
CrossRef
- Rolison D. R., Nazar L. F. Electrochemical energy storage to power 21 st century. MRS Bulletin. 2011;36(07):486-493.
CrossRef
- Ricketts B. W. Ton-That,CSelf-discharge of carbon-based supercapacitors with organic electrolytes. J. Power Sources. 2000;89:64–69.
CrossRef
- Burke A. Ultracapacitor technologies and application in hybrid and electric vehicles. Int .J. Energ. Res. 2010;34:133–151.
CrossRef
- Senthilkumar S. T.,Selvan R. K.,Lee Y. S., Melo J. S. Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J. Mater. Chem. A 1. 2013;1086–1095.
CrossRef
- Hou Y.,Chen L.,Liu P.,Kang J.,Fujita T., Chen M. Nanoporous- metal based flexible asymmetric pseudocapacitors. J.Mater.Chem. A. 2014;2:10910–10916.
CrossRef
- Inagaki M., Konno H., Tanaike O. Carbon materials for electrochemical capacitors. J. Power Sources. 2010;195:7880–7903.
CrossRef
- Simon P., Gogotsi Y. Materials for electrochemical capacitors. Nat. Mater. 2008;7:845–854.
CrossRef
- Asano Y.,Komatsu T.,Murashiro K., Hoshino K. Capacitance studies of cobalt compound nano wires prepared via electrodeposition. J.Power Sources. 2011;196:5215–5222.
CrossRef
- Zhang L. L., Zhao X. S. Carbon based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009;38(9):2520-2531.
CrossRef
- Fan L. Z.,Hu Y. S.,Maier J.,Adelhelm P.,Smarsly B and Antonietti M. High electroactivity of Polyaniline in supercapacitors by using a hierarchically porous carbon monolith as a support. Adv. Funct. Mater. 2007;17:3083–3087.
CrossRef
- Gao Y.,Chen S.,Cao D.,Wang G., Yin J. Electrochemical capacitance of Co3O4 nano wire arrays supported on nickel foam. J. Power Sources. 2010;195:1757–1760.
CrossRef
- Min-Kyu S., Cheng S., Chen H., Qin W., Kyung-Wan N., Xu S., Xiao-Qing Y., Bongiorno A., Lee J., Bai J., Trevor A. T., X Cho J and Liu M. Anomalous pseudocapacitive behavior of a nanostructured,mixed-valent manganese oxide film for electrical energy storage. NanoLett. 2012;12:3483–3490.
CrossRef
- Yuan C.,Zhang X.,Su L.,Gao B., Shen L. Facile synthesis and self- assembly of hierarchical porous NiO nano microspherical superstructures for high performance supercapacitors. J.Mater.Chem. 2009;19:5772–5777.
CrossRef
- Zhi M.,Xiang C.,Li J.,Li M., Wu N. Nanostructured carbon-metal oxide composite electrodes for supercapacitors a review. Nanoscale. 2013;5:72–88.
CrossRef
- Wang R.,Yan X.,Lang J.,Zheng Z and Zhang P. A hybrid supercapacitor based on flower-like Co(OH)2 and urchin-like VN electrode materials. J.Mater.Chem.A. 2014;2:12724–12732.
CrossRef
- Xia H.,Meng Y. S.,Yuan G.,Cui C and Lu L. Asymmetric RuO2 RuO2 super-capacitor operating at 1.6 V by using a neutral aqueous electrolyte. Electrochem. Solid State Lett. 2012;15:60–63.
CrossRef
- Zhao D.,Guo X.,Gao Y and Gao F. An electrochemical capaci- tor electrode based on porous carbon spheres hybrided with Polyaniline and nanoscale ruthenium oxide. ACS Appl. Mater. Interfaces. 2012;4:5583–5589.
CrossRef
- electrochemical supercapacitors. J. Electrochem. Soc. 1995;142:2699–2703.
- Dinh T. M., Achour A., Vizireanu S., Dinescu G., Nistor L., Armstrong K., Guay D., Pech D Hydrous RuO2/carbon nanowalls hierarchical structures for all-solid-state ultrahigh-energy-density micro-super capacitors. Nano Energy. 2014;10:288-294.
CrossRef
- Chaitra K., Sivaraman P., Vinny R. T., Bhatta U. M., Nagaraju N., Kathyayini N., High energy density performance of hydrothermally produced hydrous ruthenium oxide multi walled carbon nano tubes composite: Design of an asymmetric super capacitor with excellent cycle life. Journal of Energy Chemistry. 2016;25:627–635.
- Zheng J. P., Cygan P. J.,Jow T. R. Hydrous ruthenium oxide as an electrode material for Liu, X.R.; Pickup, P.G. Ru oxide supercapacitors with high loadings and high power and energy densities. J. Power Sources. 2008;176:410–416.
CrossRef
- Panic V., Vidakovic T., Gojkovic S., Dekanski A., Milonjic´S., Nikoli B. The properties of carbon-supported hydrous ruthenium oxide obtained from RuOxHy sol Electrochimica Acta. 2003;48:3805-3813.
CrossRef
- Hu C. C., Chen W. C., Chang K. H. How to achieve maximum utilization of hydrous ruthenium oxide for supercapacitors? J. Electrochem. Soc. 2004;151:281-290.
CrossRef
- Santos M. C., Terezo A. J., Fernandes V. C., Pereira E. C., Bulhoes L. O. S. An EQCM investigation of charging RuO2 thin films prepared by the polymeric precursor method. J. Solid State Electrochem. 2005;9:91-95.
CrossRef
- Santos M. C., Cogo L., Tanimoto S. T., Calegaro M. L.,. Bulhoes L .O. S. A nano-gravimmetric investigation of the charging processes on ruthenium oxide thin films and their effect on methanol oxidation. Appl. Surf. Sci. 2006;253:1817-1822.
CrossRef
- Hu C. C.,Chang K. H.,Lin M. C., Wu Y. T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation super capacitors. NanoLett. 2006;6:2690–2695.
CrossRef
- Dmowski W., Egami T., Swider-Lyons K. E., Love C. T., Rolison D. R. Local Atomic Structure and Conduction Mechanism of Nanocrystalline Hydrous RuO2 from X-ray Scattering. J. Phys. Chem. B. 2002;106:12677- 12683.
CrossRef
- Chang K . H., Hua C. C., Chou C. Y. Textural and pseudocapacitive characteristics of sol–gel derived RuO2·xH2O:Hydrothermal annealing vs. annealing in air. Electrochimica Acta. 2009;54:978–983.
CrossRef
- Chen I. L., Wei Y. C., Chen T. Y., Hu C. C., Lin T. L. Oxi dative precipitation of ruthenium oxide for supercapacitors: Enhanced capacitive performances by adding cetyltrimethyl ammonium bromide. Journal of Power Sources. 2014;268:430-438.
CrossRef
- Wen J., Zhoua Z. Pseudocapacitance characterization of hydrous ruthenium oxide prepared via cyclic voltammetric deposition. Materials Chemistry and Physics. 2006;98:442–446.
CrossRef
- Patake V. D., Pawar S. M., Shinde V. R., Gujar T. P., Lokhande C. D. The growth mechanism and super capacitor study of anodically deposited amorphous ruthenium oxide films. Current Applied Physics. 2010;10:99–103.
CrossRef
- Park B. O., Lokhande C. D., Park H. S., Jung K. D., Joo O. S. Performance of supercapacitor with electrodeposited ruthenium oxide film electrodes—effect of film thickness. Journal of Power Sources. 2004;134:148–152.
- Mondal S. K., Munichandraiah N. Anodic deposition of porous RuO2 on stainless steel for supercapacitor studies at high current densities. Journal of Power Sources. 2008;175:657–663.
- Kim J. H., Ahn J. H., Kang S. W., Roh J. S., Kwon S. H., Kim J. Y. Thermal stability of RuO2 thin films prepared by modified atomic layer deposition Current Applied Physics. 2012;12(2):160-163.
- Chen C. C., Chen R. S., Tsai T. Y., Huang Y. S., Tsai D. S., Tiong K. K. The growth and characterization of well aligned RuO2 nanorods on sapphire substrates. J. Phys. Condens. Matter. 2004;16 8475.
- Nam H. S., Jang K. S., Ko J. M., Kong Y. M., Kim J. D. Electrochemical capacitance of nano porous hydrous RuO2 templated by anionic surfactant. Electrochim. Acta. 2011;56:6459-6463.
- Zhang J., Ma J., Zhang L. L., Guo P., Jiang J., Zhao X. Template Synthesis of Tubular Ruthenium Oxides for Supercapacitor Applications. J. Phys. Chem. C. 2010;114:13608–13613.
- Wu X., Zeng Y., Gao H., Su J., Liu J., Zhu Z. Template synthesis of hollow fusiform RuO2·xH2O nano structure and its supercapacitor performance. J. Mater. Chem. A 1. 2013;469-472.
- Wang Y., Foo C. Y., Hoo T. K., Ng M., Lin J. Designed Smart System of the Sandwiched and Concentric Architecture of RuO2/C/RuO2 for High Performance in Electrochemical Energy Storage. Chem.-A Eur. J. 2010;16:3598-3603.
- Wu X., Xiong W., Chen Y., Lan D., Pu X., Zeng Y., Gao H., Chen J., Tong H., Zhu. High-rate super capacitor utilizing hydrous ruthenium dioxide nano tubes. Journal of Power Sources. 2015;294:88-93.
- Liu X. M., Zhang X. G. NiO-Based Composite Electrode with RuO2 for Electrochemical Capacitors. Electrochimica Acta. 2004;49(2):229-232.
- Deshmukh P. R., Bulakhe R. N., Pusawale S. N., Sartale S. D., Lokhande C. D. Polyaniline–RuO2 composite for high performance super capacitors chemical synthesis and properties. RSC Adv. 2015;5:28687-28695
- Lim J. Y., Rahman G., Chae S. Y., Lee K. Y., Kim C. S and Joo O. S. Highly stable RuO2/SnO2 nano composites as anode electrocatalysts in a PEM water electrolysis cell. Int. J. Energy Res. 2014;38:875–883.
- Yong-gang W., Xiao-gang Z. Preparation and electrochemical capacitance of RuO2/TiO2 nanotubes composites. Electrochimica Acta. 2004;49(12):1957-1962.
- Leea J. B., Jeong S. Y., Moon W. J., Seong T. Y., Ahna H. J. Preparation and characterization of electro-spun RuO2–Ag2O composite nanowires for electrochemical capacitors. Journal of Alloys and Compounds. 2011;509:4336–4340.
- Zang J., Bao S. J., Li C. M., Bian H., Cui X., Bao Q., Sun C. Q., Guo J., Lian K. Well-Aligned Cone-Shaped Nanostructure of Polypyrrole RuO2 and Its Electrochemical Supercapacitor. The Journal of Physical Chemistry C. 2008;112(38):14843-14847.
- Chen L. Y., Hou Y., Kang J. L., Hirata A., Fujita T., ChenM. W. Toward the Theoretical Capacitance of RuO2 Reinforced by Highly Conductive Nanoporous Gold Adv. Energy Mater. 2013;3:851–856.
- Li J., Wang X., Huang Q., Dai C., Gamboa S., Sebastian P. J. Preparation and characterization of RuO2·xH2O/carbon aerogel composites for super capacitors Journal of Applied Electrochemistry. 2007;37(10):1129-1135.
- Okea S., Yamamoto M., Shinohara K., Takikawa H., Xiaojun H., Itoh S., Yamaura T., Miura K., Yoshikawa K., Okawa T., Aoyagie N. Specific capacitance of electrochemical capacitor using RuO2 loading arc-soot activated carbon composite electrode. Chemical Engineering Journal. 2009;146:434–438.
- Natsuki T., Tantrakarn K., Endo M. Effects of carbon nanotube structures on mechanical properties. Applied Physics A. 2004;79(1):117-124.
- Ebbesen T. W., Lezec H. J. , Hiura H., Bennett J. W., Ghaemi H. F. Thio T. Electrical conductivity of individual carbon nano tubes. Nature. 1996;382:54–56.
- Kim J. Y., Kim K . H., Park S. H Kimet K. B. Microwave-polyol synthesis of nano crystalline ruthenium oxide nanoparticles on carbon nanotubes for electrochemical capacitors. Electrochimica Acta. 2010;55:8056–8061.
- Kim H., Popov B. N. Characterization of hydrous ruthenium oxide carbon nano composite super capacitors prepared by a colloidal method. J Power Sources. 2002;104:52-61.
- Liu X., Huber T. A., Kopac M. C., Pickup P. G. Ru oxide carbon nanotube composites for supercapacitors prepared by spontaneous reduction of Ru(VI) and Ru(VII). Electrochimica Acta. 2009;54:7141–7147.
- Park J. H., Park O. O. Morphology and electrochemical behaviour of ruthenium oxide thin film deposited on carbon paper Journal of Power Sources. 2002;109:121–126.
- Yin Y., Wang X., You Z. Integration of Ruthenium oxide-Carbon Nanotube Composites with Three-Dimensional Inter-digitated Microelectrodes for the Creation of On-Chip Supercapacitors. Int. J. Electrochem. Sci. 2017;12:3883–3906.
- Lin N., Tian J., Shan Z., Chen K., Liao W. Ruthenium nanoparticles-modified reduced graphene prepared by a green method for high-performance supercapacitor application in neutral electrolyte Electrochimica Acta. 2013;99:219–224.
- Hu C. C., Chen W. C., Chang K. H. How to Achieve Maximum Utilization of Hydrous Ruthenium Oxide for Supercapacitors. Journal of Electrochemical Society. 2004;151:281-290.
- Panic V., Vidakovic T., Gojkovic S., Dekanski A., Milonjic S., Nikolic B. The properties of carbon-supported hydrous ruthenium oxide obtained from RuOxHy sol. Electrochim. Acta. 2003;48:3805-3813.
- Hsieh T. F., Chuang C. C., Chen W. J., Huang J. H., Chen W. T., Shu C. M. Hydrous Ruthenium Dioxide Multi-Walled Carbon Nanotube Titanium Electrodes for Super capacitors. Carbon. 2012;50:1740–1747.
- Zhang C., Zhou H., Yu X., Shan D., Ye T., Huang Z., Kuang Y. Synthesis of RuO2 decorated quasi graphene nanosheets and their application in super capacitors. RSC Adv. 2014;4:11197–11205.
- Geim A. K., Novoselov K. S. The Rise of Graphene Nature Materials. 2007;6:183–191.
- Neto A. H. C., Guinea F., Peres N. M. R., Novoselov K. S., Geim A. K. The electronic properties of graphene Rev. Mod. Phys. 2009;81(109):1-55.
- Lin N., Tian J., Shan Z., Chen K., Liao W. Hydrothermal synthesis of hydrous ruthenium oxide graphene sheets for high-performance super capacitors. Electrochimica Acta. 2013;99:219–224.
- Wang R., Jia P., Yang Y., An N., Zhang Y., Wu H and Hu Z. Ruthenium Oxide Reduced Graphene Oxide Nanoribbon Composite and Its Excellent Rate Capability in Supercapacitor Application. Chin. J. Chem. 2016;34:114–122.
- Chen Y., Zhang X., Zhang D., Ma Y. graphene ruthenium oxide hybrid dispersed by polyvinylpyrrolidone. Journal of Alloys and Compounds. 2012;541:415–420.
- Yang F., Zhang L., Zuzuarregui A., Gregorczyk K., Li L., Beltrán M., Tollan C., Brede J., Rogero C., Chuvilin A., Knez M, Functionalization of Defect Sites in Graphene with RuO2 for High Capacitive Performance ACS Appl. Mater. Interfaces. 2015;7:20513−20519.
- Soin N., SinhaRoy S., Mitra S. K., Thundat T., McLaughlin J. A. Nanocrystalline ruthenium oxide dispersed Few Layered Graphene (FLG) nanoflakes as supercapacitor electrodes J. Mater. Chem. 2012;22:14944–14950.
- Wu Z. S., Wang D. W., Ren W., Zhao J., Zhou G., Li F., Cheng H. M. Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors Adv. Funct. Mater. 2010;20:3595–3602.
- Wang W., Guo S., Lee I., Ahmed K., Zhong J., Favors Z., Zaera F., Ozkan M., Ozkan C. S. Ruthenium Oxide Nanoparticles Anchored to Graphene and Carbon Nanotube Hybrid Foam for Super capacitors. Sci. Rep. 2014;4:4452. DOI:10.1038/srep04452.
H

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal