V. Madhusudhana Reddy1 , N. Kundana2
, N. Kundana2 and T. Sreekanth3
and T. Sreekanth3
1Department of Science and Humanities, Malla Reddy College of Engineering and Technology, Dhullapally, Hyderabad, T.S, India.
2BV Raju Institute of Technology, Narsapur, Medak Dist, T.S., India.
3Department of Physics, JNTUH College of Engineering Nachupally (Kondagattu), Jagtial District, T.S, India.
Corresponding author Email: srikanth2t@gmail.com
DOI : http://dx.doi.org/10.13005/msri/150103
Article Publishing History
Article Received on : 3 April 18
Article Accepted on : 20 April 18
Article Published : 21 Apr 2018
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
(PEO+KNO3+Nano Al2O3) based Composite Polymer Electrolytes (CPE) has been prepared by using solution casting technique. In this technique, Poly (ethylene oxide) (PEO) and KNO3salt were dissolved separately in methanol and they were mixed together. Nano alumina (Al2O3) (particle size ~10nm) was doped to mixed solution and stirred for 24hrs. X-ray diffraction (XRD) technique has been obtained to determine complexation of salt and polymer in composite polymer electrolytes. Ionic and electronic transference numbers of these composite polymer electrolytes has been calculated by using Wagner’s polarization technique. The DC Conductivity of these composite polymer electrolytes has been evaluated in the temperature range of 303-373 K.
KEYWORDS:
Composite polymer electrolytes; DC Conductivity; Poly (ethylene oxide); Solution casting technique; Transference numbers; X-ray diffraction
Copy the following to cite this article:
Reddy V. M, Kundana N, Sreekanth T. Investigation of XRD and Transport Properties of (PEO+KNO3+Nano Al2O3) Composite Polymer Electrolyte. Mat.Sci.Res.India;15(1)
|
Copy the following to cite this URL:
Reddy V. M, Kundana N, Sreekanth T. Investigation of XRD and Transport Properties of (PEO+KNO3+Nano Al2O3) Composite Polymer Electrolyte. Mat.Sci.Res.India;15(1). Available from: http://www.materialsciencejournal.org/?p=7220
|
Introduction
In recent times Composite Polymer Electrolytes (CPE) has been drastically explored due to its various prospective applications such as batteries, fuel cells, smart windows, solar cells, display devices, etc.1,4 These composite polymer electrolytes were used widely due to easy fabrication technique of desirable size and facilitate proper contacts with electrode and electrolyte. When nano fillers doped to polymer electrolytes then, there was an increase in the conductivity of the polymer electrolytes and also improves the mechanical strength.5 Various types of nano fillers were reported to study the performance of composite polymer electrolytes.6,21
As per the literature, it was observed that majority of the authors were reported on Li based composite polymer electrolytes and scanty reports on potassium based composite polymer electrolytes. But potassium has several advantages compared to lithium such as easy contact ability, less moisture resistant, lower price, etc.22 Therefore, in this present paper the authors reported (PEO+KNO3+NanoAl2O3) based composite polymer electrolytes. The complexation of KNO3 salt and Poly (ethylene oxide) (PEO) were confirmed by using X-ray diffraction (XRD) technique. Wagner’s polarization technique has been obtained to determine transference number of these composite polymer electrolytes. The DC conductivity of these composite polymer electrolytes has been determined in the temperature range of 303-373K.
Experimental
Solution casting technique was used for preparation composite polymer electrolytes films. In this technique Poly (ethylene oxide) (PEO) [Aldrich: Molecular Weight (4X105)] and KNO3 salt were dissolved separately in methanol and were mixed together. Nano alumina (Al2O3) (particle size ~10nm) was doped to mixed solution and stirred for 24hrs. Then after, it was casted on to polypropylene dishes, evaporated slowly at room temperature and vacuum dried carefully at 10-3 mbar to eliminate all traces of water. (PEO+KNO3+Nano Al2O3) composite polymer electrolytes films (thickness @ 100 – 150 µm) were prepared for the weight ratios (90:10), (80:20) and (70:30).23 The complexation of KNO3 and PEO have been confirmed by using X-ray diffraction setup of SIEMENS / D. 5000 X-ray diffractometer (Cu Ka radiation l = 1.5406Å).23 The ionic and electronic transference numbers of these composite polymer electrolytes were evaluated by using Wagner’s polarization technique.24 In this technique, a composite polymer electrolyte was placed between blocking electrode and non-blocking electrode and polarized by a constant DC voltage. Polarization current versus time have been obtained by using Keithley Electrometer [Model 604]. DC conductivity of these composite polymer electrolytes was determined by using lab made DC conductivity setup in the temperature range 303-373K.25
Results and Discussion
The X-ray diffraction (XRD)
The X-ray diffraction (XRD) pattern of pure Poly (ethylene oxide) (PEO), complexed PEO and pure KNO3 salts were compared together and it was shown in Fig.1. From Fig.1 following results were observed:
- In the range of 200 to 300 of XRD pattern, intensity of the peaks was lees in complexed PEO than pure PEO. Due to decrease in the degree of crystallinity of the polymer PEO by the addition of KNO3 salts and Al2O3 to PEO.
- The peaks of pure PEO exist in complexed PEO. It was happen due to coincident existence of both crystalline uncomplexed and complexed PEO. Similar reports were observed for PEO based polymer electrolytes.23,26
- Among all complexed PEO composite polymer electrolytes systems, (PEO+KNO3+Nano Al2O3) (70:30) pattern lees intensity peaks were observed due to dominant incidence of an amorphous phase.
From all the above observations, it was confirmed that KNO3 and Nano Al2O3 were complexed with PEO.
Figure 1: X-ray diffraction pattern (a) Pure PEO, (b) [PEO + KNO3 + Nano Al2O3] (80:20) (c) [PEO + KNO3+ Nano Al2O3] (70:30) and (d) KNO3.
Transference Numbers
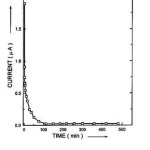
Wagner’s polarization technique was employed to determine transference numbers of tion and tele of (PEO+ KNO3+Nano Al2O3) composite polymer electrolytes systems.24,27 In this context, polarization current versus time plots were obtained for (PEO+KNO3+NanoAl2O3) (90:10), (80:20) & (70:30) composite polymer electrolytes. Current versus time plots of (PEO+ KNO3+Nano Al2O3) (70:30) was shown in Fig. 2. Transference numbers of tion and tele were calculated and tabulated in Table -1. From the obtained tion and tele values, it is clear that the conductivity occurring in these composite polymer electrolytes due to majority charge carriers i.e. ions and negligible contribution from the electronic.28
Figure 2: Polarization current versus time plot for the complexed (PEO+ KNO3+Nano Al2O3) (70:30)
DC Conductivity
The conductivity versus temperature plots were obtained in the temperature range of 303-373K for (PEO+KNO3) & (PEO+KNO3+nano Al2O3) composite polymer electrolytes systems and following facts were observed:
The conductivity versus temperature plots were obeying Arrhenius nature throughout, but showing two regions. The region–I was emerged below melting temperature (Tm) of (PEO+KNO3+nanoAl2O3) composite polymer electrolytes. The region–II was emerged above melting temperature (Tm) of (PEO+KNO3+nanoAl2O3) composite polymer electrolytes. However, in the both regions the conductivity increases with an increase of temperature, while a sudden sharp increment in the current was observed at Tm. It was occurred due to phase change from semi-crystalline phase to amorphous at Tm.29
Table 1: Transference Number data and DC Conductivity data of (PEO+KNO3+NanoAl2O3) composite polymer electrolytes systems.
|
Polymer Electrolyte
|
Transference Number
|
DC Conductivity (σ) (S.cm-1)
at RT
|
|
tion
|
tele
|
|
Pure Poly (ethylene oxide) (PEO)
|
–
|
–
|
8.35×10-10
|
|
(PEO+KNO3+ nano Al2O3) (90:10)
|
0.98
|
0.02
|
5.59xl0-8
|
|
(PEO+ KNO3+ nano Al2O3) (80:20)
|
0.98
|
0.02
|
4.88xl0-8
|
|
(PEO+ KNO3+ nano Al2O3) (70:30)
|
0.99
|
0.01
|
2.05xl0-7
|
- The conductivity of the composite polymer electrolytes increases with an increase of temperature due to hopping mechanism between coordinating sites, local structural relaxations and segmental motions of polymer.
- When amorphous region gradually increases, the polymer chain acquires faster internal modes in which bond rotations produce segmental motion. This was in turn favours the hopping inter-chain and intra-chain ion movements and the conductivity of the polymer electrolytes become high.30,31
Similar results were observed for all the systems of (PEO+KNO3) & (PEO+KNO3+nanoAl2O3) (90:10), (80:20) & (70:30). The conductivity versus temperature plots of PEO, (PEO+KNO3) (70:30) and (PEO+KNO3+nanoAl2O3) (70:30) shown in Fig.3.
The role of the Nano Al2O3
From DC Conductivity studies (Fig.3) following observations were found:
Nano filler Al2O3 doped with complexed PEO conductivity was very high compare to complexed PEO and pure Poly (ethylene oxide) (PEO). Once nano Al2O3 doped to polymer then, it reduce the crystallinity of polymer, hold back them from re-crystallization and boost the degree of amorphicity, which lead to better ionic conductivity of the polymer.32 The conductivity values of (PEO+KNO3+ nanoAl2O3) composite polymer electrolytes systems were obtained and tabulated in Table-1.
Figure 3: The temperature dependence of D.C conductivity (a) Pure PEO, (b) [PEO+KNO3] (70:30) and (c) [PEO+KNO3+nano Al2O3] (70:30)
Conclusions
Solid state (PEO+KNO3+nano Al2O3) composite polymer electrolytes have been prepared by using solution casting technique. The complexation of salt and polymer were confirmed by using X-ray diffraction (XRD) technique. XRD was obtained in the range of 200 to 300, intensity of the peaks was lees in complexed PEO than pure Poly (ethylene oxide) (PEO). The degree of crystallinity of the polymer decreases by the addition of KNO3 salt and Al2O3 to Poly (ethylene oxide) (PEO). From Wagner’s polarization technique it was clear that, conductivity occurring in these composite polymer electrolytes due to ions and negligible contribution from the electronic. The DC conductivity increase with increase of temperature due to hopping mechanism between coordinating sites, local structural relaxations and segmental motions of polymer. Nano filler Al2O3 doped with complexed PEO shown high conductivity compared to complexed PEO composite polymer electrolytes systems.
Acknowledgment
The authors express gratitude to the Principal of their colleges for their continuous support and encouragement.
Funding Source
The author declares that the funding is done by author only.
Conflict of interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
- Armand M. B. Ann. Rev. Mater. Sci. 1986;16:245.
CrossRef
- Ratner M. A.,Shriver D. F. Chem. Rev. 1988;88:109.
CrossRef
- Owen J. R., in: Lasker A. L., Chandra S (Eds.) Superionic Solids and Solid Electrolytes Recent Trends, Academic Press, New York. 1989:111.
CrossRef
- Mac J. R. C., Vincent C. A (Eds.), In: Polymer Electrolyte Reviews, Elsevier, Amsterdam. 1987.
CrossRef
- Zhang P., Yang L. C., Li L. L.,Ding M. L., Wu Y. P., Holze R. J Membr Sci. 2011;379::80-85.
CrossRef
- Capiglia C., Mustarelli P., Quartarone E., Tomasi C.,Magistris A. Solid State Ionics. 1999;118:73–79.
CrossRef
- Golodnitsky D., Ardel G., Peled E. Solid State Ionics. 2002;147:141–155.
CrossRef
- Jayathilaka P. A. R. D., Dissanayake M. A. K. L.,Albinsson I., Mellander B. E., Electrochimica Acta. 2002;47:3257-3268.
CrossRef
- Pitawala H. M. J. C., Dissanayake M. A. K. L., Seneviratne V. A. Solid State Ionics. 2007;178:885–888.
CrossRef
- Rafie M. J.,Hooi O. S., Ibrahim S., Mariah S. M. Y., Yin T. H. Solid State Ionics. 2011;196:41–47.
CrossRef
- Masoud E. M., El-Bellihi A. A., Bayoumy W. A., Mousa M. A. Materials Research Bulletin. 2013;48::1148–1154.
CrossRef
- Tominaga Y.,Asai S., Sumita M., Panero S., Scrosati B. Journal of Power Sources. 2005;146:402–406.
CrossRef
- Suriani Ibrahim, Siti Mariah Mohd Yasin, Ng Meng Nee, Roslina Ahmad, Mohd Rafie Johan, Journal of Non-Crystalline Solids. 2012;358:210–216.
CrossRef
- Jong-Wook Kim, Kwang-Sun Ji, Jae-Pil Lee, Jong-Wan Park, Journal of Power Sources. 2003;119–121:415–421.
CrossRef
- M. Jaipal Reddya, Peter P. Chu, J. Siva Kumar, U.V. Subba Rao, Journal of Power Sources. 2006;161:535–540.
CrossRef
- K.Vignarooban, M.A.K.L. Dissanayake, I. Albinsson, B.E. Mellander, Solid State Ionics. 2014;266::25–28.
CrossRef
- Peter P. Chu, M. Jaipal Reddy, H.M. Kao, Solid State Ionics. 2003;156:141– 153.
CrossRef
- Thomas K.J. Köster, Leo van Wüllen, Solid State Ionics. 2010;181:489–495.
CrossRef
- E. Matykina, R. Arrabal, M. Mohedano, B. Mingo, J. Gonzalez, A. Pardo, M. C. Merino, Trans. Nonferrous Met. Soc. China. 2017;27:1439−1454.
CrossRef
- Emad M. Masoud, A.A. El-Bellihi, W.A. Bayoumy, M.A. Mousa, Journal of Alloys and Compounds. 2013;575:223–228.
CrossRef
- LongChen, YutaoLi, Shuai-PengLi, Li-ZhenFan, Ce-WenNan, John B.Goodenough, Nano Energy. 2013;46:2018:176-184.
- T. Sreekanth, M. Jaipal Reddy, S. Subramanyam, U.V. Subba Rao, Materials Science and Engineering B, 64. 1999:107–112.
CrossRef
- T. Sreekanth, Journal of the Korean Physical Society. April 2013;62(8):1129–1133.
CrossRef
- J.B. Wagner and C. Wagner, J. of Chem. Phys. 1957;26:1597.
CrossRef
- Andrea Boschin, Patrik Johansson, ElectrochimicaActa. 2015;175:124-133.
CrossRef
- S.A. Hashmi, K.K. Ajay Kummar, Maurya, S. Chandra, J. Phys. D. Appl. Phys. 23:1990:1307.
CrossRef
- T. Sreekanth, International Journal of Engineering Research and Application. 2017;6(7):47-51.
CrossRef
- V. Madhusudhana Reddy, N. Kundana, T. Sreekanth, International Journal of Current Engineering and Scientific Research. 2017;12(4):59-64.
CrossRef
- T. Sreekanth, M. Jaipal Reddy, U.V. Subba Rao, Journal of Power Sources. 2001;93:268-272
CrossRef
- M.A. Ratner, In: Polymer Electrolyte Reviews-1, (eds.) J.R. Mac Callum and C.A. Vincent (Elsevier, London, New York.1987;173.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , N. Kundana2
, N. Kundana2 and T. Sreekanth3
and T. Sreekanth3
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal

![Fig.1. X-ray diffraction pattern (a) Pure PEO, (b) [PEO + KNO3 + Nano Al2O3] (80:20) (c) [PEO + KNO3+ Nano Al2O3] (70:30) and (d) KNO3.](http://www.materialsciencejournal.org/wp-content/uploads/2018/04/Vol15_No1_Inv_Mad_Fig1-150x150.jpg)
![Fig.3. The temperature dependence of D.C conductivity (a) Pure PEO, (b) [PEO+KNO3] (70:30) and (c) [PEO+KNO3+nano Al2O3] (70:30)](http://www.materialsciencejournal.org/wp-content/uploads/2018/04/Vol15_No1_Inv_Mad_Fig3-150x150.jpg)

