WC (Tungsten Carbide) : A Novel Material for Electrochemical Energy Conservation and Storage
Anjali Bisht, Tanvi Tyagi, Rekha, Sameena Mehtab , Shreen Masroor and MGH Zaidi*
, Shreen Masroor and MGH Zaidi*
Department of Chemistry, College of Basic Science and Humanities, G.B. Pant University of Agriculture and Technology,
Pantnagar, U.S Nagar-263145, Uttarakhand, India
Corresponding Author Email: mgh_zaidi@yahoo.com
DOI : http://dx.doi.org/10.13005/msri/150203
Article Publishing History
Article Received on : 19-June-2018
Article Accepted on : 08-August-2018
Article Published : 09 Aug 2018
Plagiarism Check: Yes
Article Metrics
Copy the following to cite this article:
Bisht A, Tyagi T, Rekha, Mehtab S, Masroor S, Zaidi M. WC (Tungsten Carbide) : A Novel Material for Electrochemical Energy Conservation and Storage. Mat.Sci.Res.India;15(2)
|
Copy the following to cite this URL:
Bisht A, Tyagi T, Rekha, Mehtab S, Masroor S, Zaidi M. WC (Tungsten Carbide) : A Novel Material for Electrochemical Energy Conservation and Storage. Mat.Sci.Res.India;15(2). Available from: http://www.materialsciencejournal.org/?p=8190
|
The present report deals with development and electrochemical investigation of metal carbide based energy storage systems with WC (60 nm) as a model electrode material. This is in view to extend the understanding towards tailoring and designing other transition metal carbides based electrochemical energy conservation and storage systems with desired activity and stability.
Nano structured metal carbides has recently received accelerated interest as materials for enhanced electrochemical energy storage due to their appealing electrical conductivity, chemical, thermal and electrochemical stabilities.1,2 This has been in view to develop the inexpensive and durable capacitors and batteries to meet out the power shortage in domestic, industrial and defense sectors.3 The early applications of metal carbides in development of tools, industrial catalysis, and protective coatings in fusion reactors 4-6 has now turned into fabrication of electrodes for batteries and fuel cells.7,8
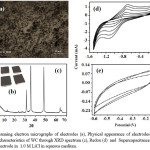
Figure 1: Scanning electron micrographs of electrodes (a), Physical appearance of electrodes (b) with retained characteristics of WC through XRD spectrum (c), Redox (d) and Supercapacitance behavior (e) of electrode in 1.0 M LiCl in aqueous medium.
Recently, tungsten carbide has also been intensively studied in the field of electrocatalyst and fuel cell material due to its high electrical conductivity, reasonable cost, platinum like catalytic behavior, good hardness, excellent selectivity and stability under harsh environment.10,11 Tungsten carbide generally appears as WC, W2C and WC1-x.12, 13 The better tolerance of WC enhances its importance as electrode materials for batteries and fuel cells.14 The characteristic hexagonal structure of WC involves small carbon atoms distributed into interstitial space of a densely packed host lattice. This imparts high melting point (2600 °C), hardness, thermal and electrical conductivity. The electrochemical behavior of tungsten carbide is strongly dependent on its surface composition and condition. Thus, carbon content of surface layers is decisive for the electrochemical performance of WC.5
The pioneer efforts have been made towards fabrication and investigation of electrochemical behavior of electrodes derived through dispersion of WC into graphite matrix in presence of an ionic polymer as binder. Scanning electron microscopy reveals high quality dispersion of WC into graphite [Fig.1(a)]. The electrode preserves the XRD peaks of WC in graphite matrix [Fig.1(c)]. Electrodes display well electrochemical behavior under three electrode assembly systems in LiCl (1.0M) @ 0.2 V/s under potential window of 0.0 to -1.5 V [Fig.1(d)]. This has raised the anodic peak current up to 1.87 mA and cathodic peak current to -2.92 mA. The increase in the peak current with scan rate attributes to good reversibility of electrodes during the charge-discharge process. Further, the polarization of the electrode under high scan rate attributes to the increased potential difference between anodic and cathodic peaks.16
Conclusion
Electrodes display enhanced stability and cyclibility up to 0.1 V/s without noticeable decomposition indicating their suitability as electrode materials.Under optimized composition, potential window and scan rate, WC based electrodes of the present study delivers supercapacitance up to 235 F/g in LiCl (1M) [Fig.1(e)].
Acknowledgement
The student fellowship granted to Anjali Bisht by GB pant University of Agriculture & Technology is acknowledged.
References
- Xiao Y., Hwang J. Y.,Sun Y. K. Transition metal carbide-based materials synthesis and applications in electrochemical energy storage. J. Mater. Chem. A. 2016;4:10379-10393.
CrossRef
- Zhong Y.,Xia X., Shi F.,Zhan J., Tu J., Fan H. J. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2016;3:37-39.
CrossRef
- Xie Y., Naguib M., Mochalin V. N.,Barsoum M.W., Gogotsi Y., Yu X., Nam K. W., X. Q., Yang A. I. Kolesnikov, P. R. Kent, Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 2014;136:6385-6394.
CrossRef
- Flaherty D. W., May R. A., Berglund S. P., Stevenson K. J., Mullins C. B. Low temperature synthesis and characterization of nanocrystalline titanium carbide with tunable porous architectures. Chem. Mater. 2009;22:319-329.
CrossRef
- Wang Y. J., Wilkinson D. P.,Zhang J. Noncarbon support materials for polymer electrolyte membrane fuel cell electrocatalysts. Chem. Rev. 2011;111:7625-7651.
CrossRef
- Wu M., Lin X., Hagfeldt A.,Ma T. Low cost molybdenum carbide and tungsten carbide counter electrodes for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011;50:3520-3524.
CrossRef
- Ham D. J.,Lee J. S. Transition metal carbides and nitrides as electrode materials for low temperature fuel cells. Energies. 2009;2:873-899.
CrossRef
- Xie Y., Naguib M., Mochalin V. N., Barsoum M. W., Gogotsi Y., Yu X., Nam K. W., Yang X. Q., Kolesnikov A. I., Kent P. R. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 2014;136:6385-6394.
CrossRef
- A.S. Kurlov, A.I. Gusev, Tungsten carbides: structure, properties and application in hardmetals. Springer Science & Business Media. 2013;184.
CrossRef
- Xu R. D., Huang L. P., Zhou J .F., Zhan P., Guan Y. Y., Kong Y. Effects of tungsten carbide on electrochemical properties and microstructural features of Al/Pb-PANI-WC composite inert anodes used in zinc electrowinning. Hydrometallurgy. 2012;125:8-15.
CrossRef
- Zeng J., Yuan D., Liu Y., Chen J., Tan S. Synthesis of tungsten carbide nanocrystal and their electrochemical properties. Frontiers of Chemistry in China. 2009;4:127-131.
CrossRef
- Zellner M. B., Chen J. G. Surface science and electrochemical studies of WC and W2C PVD films as potential electrocatalysts. Catal. Today. 2005;99(3-4):299-307.
CrossRef
- Suetin D. V., Shein I. R., Ivanovski A. L. Structural, electronic properties and stability of tungsten mono-and semi-carbides: A first principles investigation. J. Phys. Chem. Solids. 2009;70:64-71.
CrossRef
- Liu W., Soneda Y., Kodama M., Yamashita J and Hatori H. Low-temperature preparation and electrochemical capacitance of WC/carbon composites with high specific surface area. Carbon. 2007;45:2759-2767.
CrossRef
- Ross P. N. Jr., Macdonald J., Stonehart P. Surface composition of catalytically active tungsten carbide (WC). J Electroanal Chem Interfacial Electrochem. 1975;63:450-455.
CrossRef
- Raj R. P., Ragupathy P.,Mohan S. Remarkable capacitive behavior of a Co3O4 polyindole composite as electrode material for supercapacitor applications. J. Mater. Chem. A. 2015;3:24338-24348.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Shreen Masroor and MGH Zaidi*
, Shreen Masroor and MGH Zaidi*
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal