Ayse KALEMTAS*
Department of Metallurgical and Materials Engineering, Bursa Technical University, 16310 Bursa, Turkey.
Corresponding Author E-mail: ayse.kalemtas@btu.edu.tr
DOI : http://dx.doi.org/10.13005/msri/160107
Article Publishing History
Article Received on : 19-12-2018
Article Accepted on : 24-01-2019
Article Published : 25 Jan 2019
Plagiarism Check: Yes
Reviewed by: Rajesh Kumar Meena
Second Review by: Abrar Ahamed
Final Approval by: Jit Satyabrata
Article Metrics
ABSTRACT:
In the current study, sodium borate-bonded highly open porous ceramics successfully produced by starch consolidation technique. Open porous ceramic production was carried out by using an economical grade a-Si3N4, corn starch, CC31 commercial-grade kaolin, and borax decahydrate (Na2B4O7.10H2O). Borax decahydrate was used as a sintering aid in the system and total ceramic (a-Si3N4 + CC31): borax decahydrate ratio was kept constant at 5:1. Sintering studies of the shaped samples carried out in an air atmosphere at a relatively low sintering temperature, 1100°C, for one hour. Scanning electron microscopy investigations of the porous ceramic samples revealed that due to the high amount of borax based sintering additive a significant amount of liquid phase formed during the sintering process of the designed ceramics. Highly open porous(~66-74%) and lightweight(~0.64-0.83 g/cm3) ceramics were produced via starch consolidation technique and low-temperature sintering at atmospheric conditions.
KEYWORDS:
Low-Temperature Sintering; Porous Ceramics; Sodium Borate; Starch Consolidation
Copy the following to cite this article:
Kalemtas A. Low-Temperature Sintering of Porous Ceramics Via Sodium Borate Addition. Mat.Sci.Res.India;16(1).
|
Copy the following to cite this URL:
Kalemtas A. Low-Temperature Sintering of Porous Ceramics Via Sodium Borate Addition. Mat.Sci.Res.India;16(1). Available from: https://bit.ly/2FNMjkX
|
Introduction
Recently porous ceramics, polymers, metals, and composites are presently on focus of many engineering research studies as well as industrial development studies.1-10 Porous ceramic materials exhibit many distinct characteristics when compared with the metals and polymers, such as high hardness, specific surface area, chemical inertness, wear resistance, thermal shock resistance, corrosion resistance, excellent mechanical and chemical stability at elevated temperatures, low thermal expansion coefficient, and low density.1,11,12 Due to these characteristics properties porous ceramic materials are promising materials for various functional and structural applications. The characteristics of the pores such as pore size and morphology are key factors that determines applications of these porous materials. The potential applications of porous ceramics are separation materials,13 sensors,14 catalyst supports,15-17 filtration of fluids,18 heat insulating19 and implantable bioceramics,20-22 etc.
Some of the conventional processing methods used for the fabrication of porous ceramics are freeze casting,20,22-24 direct foaming method,25-28 starch consolidation,29-33 polymeric sponge method,34-37 and adding pore former agents (such as starch, phosphoric acid, and carbon)38-41 and gel−casting42,43 method. All these processing methods have its advantages and limitations. Among these techniques, starch consolidation method is a novel promising near net−shaping and cost−effective method used for the production of various porous ceramic materials.29,32 Furthermore, starch consolidation does not require any specialized equipment, and it is relatively a simple processing method. This method provides a very systematic approach to control the microstructural development of the porous ceramics by designing the content of the ceramic slurry including the solid loading ratio, amount and type of the used starch, and by the rate of sintering achieved during the sintering step. Due to these advantages, starch consolidation method gained considerable attention, especially in the last two decades.31,32,44-51
The objective of this study was to produce highly porous ceramics via starch consolidation technique. In this study, starch was used for both as pore former and gelling agent. Starch is a well-known readily available, environmentally friendly, low-cost polysaccharide synthesized by plants and it has relatively low burn out temperature without generation of any toxic materials. Samples were pressureless sintered under the atmospheric conditions at 11000C for one hour. Microstructure development, open porosity, bulk density, and phase content of the fabricated porous ceramics were investigated.
Experimental Procedure
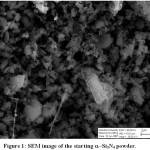
Open porous ceramic production was carried out by using an economical grade α−Si3N4, corn starch, CC31 commercial-grade kaolin, and borax decahydrate (Na2B4O7.10H2O). Borax decahydrate was used as a sintering aid in the system and total ceramic (α−Si3N4 + CC31):boraxdecahydrate ratio was kept constant at 5:1. Scanning electron microscopy (SEM) image of the starting fine-grained α−Si3N4 ceramic powder is given in Fig. 1.
XRD pattern of the used CC31 commercial-grade kaolin powder is given in Fig. 2. XRD analysis result of the CC31 showed mainly a typical diffraction pattern of kaolinite phase with some illite and free quartz as an impurity.
Designed compositions (Table 1) and deionized water mixed via wet milling process by using a planetary ball mill to achieve a homogenous slurry. Prepared ceramic slurries poured into plastic molds and then placed into an oven that was 800C. One hour dwell time was applied to achieve gelatinization of the starch content of the samples. After the gelatinization process, obtained green samples dried at room temperature for at least 24 hours.
Table 1: Designed compositions
|
Designation
|
Si3N4
g
|
Borax Decahydrate
g
|
CC31 commercial–grade kaolin
g
|
Starch
g
|
|
S1
|
70
|
14
|
1.75
|
30
|
|
S2
|
60
|
12
|
1.50
|
40
|
|
S3
|
50
|
10
|
1.25
|
50
|
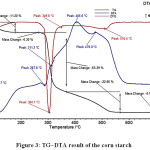
A simultaneous thermogravimetry and differential thermal analyzer (TG/DTA, Netzsch STA 449F3) was used to determine binder burn out process conditions for the starch removal process. Investigations were done by TG/DTA in an air atmosphere at a constant heating rate of 100C/minute. It was determined that the corn starch was removed entirely from the structure just below 5600C (Fig. 3).
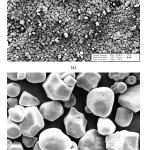
Binder burn out studies of the ceramic green bodies were done at 6500C for one hour under an air atmosphere. Applied binder burn out process heating rate was 10C/minute. Sintering studies of the prepared samples carried out with a heating rate of 50C//minute up to 11000C/ under air atmosphere. Archimedes displacement method used to determine bulk density and open porosity amount of the fabricated porous ceramic samples. Phase content of the samples was determined via X-ray diffraction (XRD) analysis. XRD investigations were performed by using monochromatic Cu-Kα radiation. Before XRD analysis, sintered porous ceramic samples crushed and sieved down to<63 μm. Starting materials and fracture surface of the sintered porous ceramic samples investigated by using scanning electron microscopy (SEM). SEM micrographs of the used corn starch are given in Fig. 4. It was determined that the average particle size of the corn starch particles was approximately 15-20 µm (Fig. 4).
Results and Discussion
Bulk density and open porosity measurement results of the sintered ceramics are given in Table 2. Depending on the increased starch content of the samples, open porosity amount of the samples slightly increased, whereas the bulk density of the samples decreased. It was determined that highly open porous (∼66−74%) and light-weight (∼0.64−0.83 g/cm3)) ceramic samples were produced.
Table 2: Open porosity and bulk density measurement results
|
Composition
|
Bulk Density
g/cm3
|
Open Porosity
%
|
|
S1
|
0.81 ± 0.02
|
66.7 ± 1.0
|
|
S2
|
0.77 ± 0.02
|
68.7 ± 1.0
|
|
S3
|
0.66 ± 0.02
|
73.0 ± 0.5
|
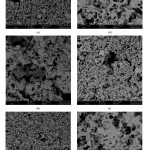
Scanning electron microscopy investigations of the sintered porous ceramic samples revealed that a three-dimensional interconnected porous network structure obtained for all samples (Fig. 5). It was observed that there were two different sizes of pores, big pores (10-50 µm) developed by the burn out of the starch particles and fine pores (smaller than a few µm) formed between the ceramic particles (Fig. 5). Microstructural analysis revealed that a high amount of liquid phase formed in all samples during the heat treatment process. Due to the sintering process performed under atmospheric conditions, starting α−Si3N4 powder can readily give a reaction with the oxygen in air and silica can form in the samples. Besides, some reactions may take place between silica and borax decahydrate sintering additive in the system, which may result in a liquid phase formation that has a low melting temperature.
Figure 5: SEM micrographs of the porous (a–b) S1, (c–d) S2 and (e–f) S3 samples at 1000X and 5000X magnifications
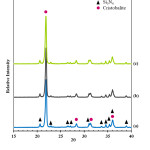
Phase analysis of the fabricated porous ceramic samples is given in Fig. 6. XRD results showed that cristobalite phase formed in all the samples due to the applied heat treatments under the atmospheric conditions. It was determined that starting α−Si3N4 powder significantly consumed during the sintering process via reacting with the oxygen in the air atmosphere. It is believed that a liquid phase formation that has a low melting temperature took place due to borax containing sintering additive and this liquid phase formation has a considerable effect on the development of the cristobalite formation.
Figure 6: X–ray diffraction patterns of the (a) S1, (b) S2 and (c) S3 ceramics
Conclusion
In this study, natural corn starch was successfully used both as a pore and green body forming agent to achieve highly open porous (∼66−74%) and lightweight (∼0.64−0.83 g/cm3) sodium borate-bonded ceramics.
Starting α−Si3N4 ceramic content of the samples considerably consumed during the sintering process via reacting with the oxygen in the air atmosphere. XRD analysis of the samples revealed that a considerable amount of cristobalite phase formed in all samples due to the sintering step under atmospheric conditions and used borax decahydrate (Na2B4O7.10H2O) sintering additive.
It was determined that highly open porous structures achieved for all the sintered ceramic samples. There were two different sizes of pores in the microstructure, big pores (10-50 µm) developed by the burn out of the starch particles and fine pores (smaller than a few µm) formed just between the ceramic particles.
Acknowledgment and Funding Source
The author wishes to thank “The Scientific and Technological Research Council of Turkey” (TÜBİTAK Project Number: 114M510) for the financial support of this study.
Conflict of Interest
The author declares that she has no conflict of interest regarding publication of this article.
References
- Studart A.R., Gonzenbach U.T., Tervoort E., Gauckler L.J. Processing routes to macroporous ceramics: a review, Journal of the American Ceramic Society (2006); 89(6): 1771-1789.
CrossRef
- Zhao C. Review on thermal transport in high porosity cellular metal foams with open cells, Int J Heat Mass Tran (2012); 55(13-14): 3618-3632.
CrossRef
- Colombo P. Conventional and novel processing methods for cellular ceramics, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences (2005); 364(1838): 109-124.
- Yuan W., Tang Y., Yang X., Wan Z. Porous metal materials for polymer electrolyte membrane fuel cells–a review, Applied Energy (2012); 94: 309-329.
CrossRef
- Kitagawa S, Kitaura R., Noro S.i. Functional porous coordination polymers, Angewandte Chemie International Edition (2004); 43(18): 2334-2375.
CrossRef
- Ng L.Y, Mohammad A.W, Leo C.P, Hilal N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review, Desalination (2013); 308: 15-33.
CrossRef
- Banhart J. Manufacture, characterisation and application of cellular metals and metal foams, Progress in materials science (2001); 46(6): 559-632.
CrossRef
- Smith B., Szyniszewski S., Hajjar J., Schafer B., Arwade S. Steel foam for structures: A review of applications, manufacturing and material properties, Journal of Constructional Steel Research (2012); 71: 1-10.
CrossRef
- Wang J., Chow W. A brief review on fire retardants for polymeric foams, Journal of applied polymer science (2005); 97(1): 366-376.
CrossRef
- Lefebvre L.P., Banhart J., Dunand D.C. Porous metals and metallic foams: current status and recent developments, Advanced Engineering Materials (2008); 10(9): 775-787.
CrossRef
- Sun Z., Fan J., Yuan F. Three-dimensional porous silica ceramics with tailored uniform pores: prepared by inactive spheres, Journal of the European Ceramic Society (2015); 35(13): 3559-3566.
CrossRef
- Colombo P. Conventional and novel processing methods for cellular ceramics, Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences (2006); 364(1838): 109-124.
- Kitaoka S., Matsushima Y., Chen C., Awaji H. Thermal cyclic fatigue behavior of porous ceramics for gas cleaning, Journal of the American Ceramic Society (2004); 87(5): 906-913.
CrossRef
- Bouwmeester H.J., Burggraaf A.J. Dense ceramic membranes for oxygen separation, The CRC handbook of solid state electrochemistry (1997); 481.
- Moene R., Makkee M., Moulijn J. High surface area silicon carbide as catalyst support characterization and stability, Applied Catalysis A: General (1998); 167(2): 321-330.
CrossRef
- Pham-Huu C., Bouchy C., Dintzer T., Ehret G., Estournes C., Ledoux M.J. High surface area silicon carbide doped with zirconium for use as catalyst support. Preparation, characterization and catalytic application, Applied Catalysis A: General (1999); 180(1-2): 385-397.
CrossRef
- Ismagilov Z., Shkrabina R., Koryabkina N., Kirchanov A., Veringa H., Pex P. Porous alumina as a support for catalysts and membranes. Preparation and study, Reaction Kinetics and Catalysis Letters (1997); 60(2): 225-231.
CrossRef
- Jo Y., Hutchison R., Raper J.A. Characterization of ceramic composite membrane filters for hot gas cleaning, Powder technology (1997); 91(1): 55-62.
CrossRef
- Litovsky E., Shapiro M., Shavit A. Gas pressure and temperature dependences of thermal conductivity of porous ceramic materials: Part 2, refractories and ceramics with porosity exceeding 30%, Journal of the American Ceramic Society (1996); 79(5): 1366-1376.
CrossRef
- Yoon B.H., Koh Y.H., Park C.S., Kim H.E. Generation of large pore channels for bone tissue engineering using camphene‐based freeze casting, Journal of the American Ceramic Society (2007); 90(6): 1744-1752.
CrossRef
- Yoon B.-H., Choi W.-Y., Kim H.-E., Kim J.-H., Koh Y.-H. Aligned porous alumina ceramics with high compressive strengths for bone tissue engineering, Scripta Mater (2008); 58(7): 537-540.
CrossRef
- Deville S., Saiz E., Tomsia A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering, Biomaterials (2006); 27(32): 5480-5489.
CrossRef
- Deville S. Freeze‐casting of porous ceramics: a review of current achievements and issues, Advanced Engineering Materials (2008); 10(3): 155-169.
CrossRef
- Araki K., Halloran J.W. Porous ceramic bodies with interconnected pore channels by a novel freeze casting technique, Journal of the American Ceramic Society (2005); 88(5): 1108-1114.
CrossRef
- Hammel E., Ighodaro O.-R., Okoli O. Processing and properties of advanced porous ceramics: An application based review, Ceram Int (2014); 40(10): 15351-15370.
CrossRef
- Mao X., Wang S., Shimai S. Porous ceramics with tri-modal pores prepared by foaming and starch consolidation, Ceram Int (2008); 34(1): 107-112.
CrossRef
- Pokhrel A., Seo D.N., Lee S.T., Kim I.J. Processing of porous ceramics by direct foaming: a review, Journal of the Korean Ceramic Society (2013); 50(2): 93-102.
CrossRef
- Fukushima M., Colombo P. Silicon carbide-based foams from direct blowing of polycarbosilane, Journal of the European Ceramic Society (2012); 32(2): 503-510.
CrossRef
- Lyckfeldt O., Ferreira J. Processing of porous ceramics by ‘starch consolidation’, Journal of the European Ceramic Society (1998); 18(2): 131-140.
CrossRef
- Gregorová E., Pabst W. Process control and optimized preparation of porous alumina ceramics by starch consolidation casting, Journal of the European Ceramic Society (2011); 31(12): 2073-2081.
CrossRef
- Gregorová E., Pabst W. Porosity and pore size control in starch consolidation casting of oxide ceramics—achievements and problems, Journal of the European Ceramic Society (2007); 27(2-3): 669-672.
CrossRef
- Alves H., Tarı G., Fonseca A., Ferreira J. Processing of porous cordierite bodies by starch consolidation, Mater Res Bull (1998); 33(10): 1439-1448.
CrossRef
- Rodríguez‐Lorenzo L., Vallet‐Regí M., Ferreira J. Fabrication of porous hydroxyapatite bodies by a new direct consolidation method: starch consolidation, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials (2002); 60(2): 232-240.
CrossRef
- Kalemtas A., Ozey N., Aydin M.T.A. Processing of layered porous mullite ceramics, Journal of the Australian Ceramic Society (2018); 54(3): 545-555.
CrossRef
- Juillerat F.K., Engeli R., Jerjen I., Sturzenegger P.N., Borcard F., Juillerat-Jeanneret L., Gerber-Lemaire S., Gauckler L.J., Gonzenbach U.T. Synthesis of bone-like structured foams, Journal of the European Ceramic Society (2013); 33(9): 1497-1505.
CrossRef
- Kara H., Ramesh R., Stevens R., Bowen C.R. Porous PZT ceramics for receiving transducers, IEEE transactions on ultrasonics, ferroelectrics, and frequency control (2003); 50(3): 289-296.
CrossRef
- Koç N., Timuçin M., Korkusuz F. Fabrication and characterization of porous hydroxyapatite and biphasic calcium phosphate ceramic as bone substitutes, Key Engineering Materials, Trans Tech Publ, 2004; 949-952.
- Kalemtas A., Topates G., Özcoban H., Mandal H., Kara F. Janssen R. Mechanical characterization of highly porous β-Si3N4 ceramics fabricated via partial sintering & starch addition, Journal of the European Ceramic Society (2013); 33(9): 1507-1515.
CrossRef
- Dı́az A., Hampshire S. Characterisation of porous silicon nitride materials produced with starch, Journal of the European Ceramic Society (2004); 24(2): 413-419.
CrossRef
- Gregorová E., Pabst W. Porous ceramics prepared using poppy seed as a pore-forming agent, Ceram Int (2007); 33(7): 1385-1388.
CrossRef
- Živcová Z., Černý M., Pabst W., Gregorová E. Elastic properties of porous oxide ceramics prepared using starch as a pore-forming agent, Journal of the European Ceramic Society (2009); 29(13): 2765-2771.
CrossRef
- Yang Z.H., Chen N., Qin X.M. Fabrication of Porous Al2O3 Ceramics with Submicron-Sized Pores Using a Water-Based Gelcasting Method, Materials (2018); 11(9).
CrossRef
- Sepulveda P., Binner J., Rogero S., Higa O., Bressiani J. Production of porous hydroxyapatite by the gel‐casting of foams and cytotoxic evaluation, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials (2000); 50(1): 27-34.
CrossRef
- Zhu T.L., Wang Y.F. Alumina Ceramics Fabricated by in-situ Consolidation of Pre-gelling Starch, J Wuhan Univ Technol (2018); 33(3): 758-766.
CrossRef
- Zang W.J., Jia T., Dong X., Liu J.C., Du H.Y., Hou F., Guo A.R. Preparation of homogeneous mullite-based fibrous ceramics by starch consolidation, Journal of the American Ceramic Society (2018); 101(7): 3138-3147.
CrossRef
- Sandoval M.L., Talou M.H., Martinez A.G.T., Camerucci M.A., Gregorova E., Pabst W. Porous cordierite-based ceramics processed by starch consolidation casting-Microstructure and high-temperature mechanical behavior, Ceram Int (2018); 44(4): 3893-3903.
CrossRef
- Kanlai K., Wasanapiarnpong T., Wiratphinthu B., Serivalsatit K. Starch consolidation of porous fused silica ceramics, J Met Mater Miner (2018); 28(1): 71-76.
- Chen Z.W., Xu G.G., Cui H.Z., Zhang X.Y., Zhan X.Y. Preparation of porous Al2O3 ceramics by starch consolidation casting method, Int J Appl Ceram Tec (2018); 15(6): 1550-1558.
CrossRef
- Xu G.G., Chen Z.W., Zhang X.Y., Cui H.Z., Zhang Z.H., Zhan X.Y. Preparation of porous Al2TiO5-Mullite ceramic by starch consolidation casting and its corrosion resistance characterization, Ceram Int (2016); 42(12): 14107-14112.
CrossRef
- Ahmed Y.M.Z., Ewais E.M.M., El-Sheikh S.M. Potato starch consolidation of aqueous HA suspension, J Asian Ceram Soc (2015); 3(1): 108-115.
CrossRef
- Ahmed Y.M.Z., Ewais E.M.M., El-Sheikh S.M. Effect of dispersion parameters on the consolidation of starch-loaded hydroxyapatite slurry, Process Appl Ceram (2014); 8(3): 127-135.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal