M. Sathish Kumar1* , M. Saroja2 and M. Venkatachalam2
, M. Saroja2 and M. Venkatachalam2
1Department of Electronics, Nehru Arts and Science, Coimbatore, Tamilnadu, India
2Department of Electronics, Erode Arts and Science, Coimbatore, Tamilnadu, India
Corresponding Author Email: mskeasc@gmail.com
DOI : http://dx.doi.org/10.13005/msri/160210
Article Publishing History
Article Received on : 04 Apr 2019
Article Accepted on : 18 May 2019
Article Published : 20 May 2019
Plagiarism Check: Yes
Reviewed by: Krishna Gudikandula
Second Review by: Rama Lingam Final Approval by: Jit Satyabrata
Final Approval by: Jit Satyabrata
Article Metrics
ABSTRACT:
The development of biomedical electronics, biosynthesis of ZnS nanoparticles(NPs) much attracted researchers, due to an eco-friendly and cost-effective routes forsynthesisZnSnanoparticles. In this present work, ZnS NPswas synthesized by using acalypha indica and curcuma longa plant extract using chemical co-precipitation method. The structural, morphological, element composition of biosynthesisZnS NPs was characterized by XRD, SEM and EDAX respectively. Optical and photoluminescence (PL) properties were evaluated by UV Visible spectroscopy. The formation of inhibition zone diameter against human pathogenic microorganisms was screened by in vitro disc diffusion method. From this investigation formation of inhibition zones clearly shows biosynthesizedZnS NPs have high antimicrobial activity against tested organisms, especially curcuma longa plant extract mediated ZnS NPs was formed maximum inhibition against all the tested microorganism.
KEYWORDS:
Acalypha Indica; Antimicrobial Activity; Bio-Fabrication; Curcuma Longa; Zinc Sulfide
Copy the following to cite this article:
Sathish Kumar M, Saroja M, Venkatachalam M. Acalypha indica and Curcuma longa plant extracts mediated ZnS nanoparticels. Mat. Sci. Res. India;16 (2).
|
Copy the following to cite this URL:
Sathish Kumar M, Saroja M, Venkatachalam M. Acalypha indica and Curcuma longa plant extracts mediated ZnS nanoparticels. Mat. Sci. Res. India;16 (2). Available from: https://bit.ly/2LUBnWM
|
Introduction
In the last two decades, fabrication of inorganic semiconductor materials widely attracted researchers because of their excellent chemical and physical properties. Among all II-VI group semiconductors, ZnS having cubic and hexagonal crystal structure with large band gap (~3.63 eV)and high refractive index in the visible range.1-4 The various synthesized method has been used to fabricate ZnS such as thermal decomposition, sol-gel technique, microemulsion method hydrothermal method, CVD, CBD, Microwave-assisted, sonochemical method, soft chemistry method, etc.5-12 The bio-fabricated nanoparticles were used various applications like solar cells, field emitters, ultraviolet light-injection lasers, emitting diodes, electroluminescent devices, infrared windows, spintronics, flat-panel displays, optoelectronics, sensors, photocatalysis and antimicrobial activity.13-22 Acalypha indica and curcuma longa is good medicinal plant and widely used novel drug medicine, green pharmacy due to the antimicrobial activity23 In the recent time, biosynthesis of ZnS NPs using different biological extracts were reported.24-25 Biosynthesized ZnS NPs from various plant extracts has been already reported by our earliest reports. BiosynthesizedZnS NPsexhibits rapid generation of electron-hole pairs by photo-excitation and the highly negative reduction potentials of excited electrons have been generated high electron-hole pair when treated under UV visible light, and it increases antimicrobial properties.26-27 In this present investigation, we reported structural optical and antimicrobial activity of ZnS NPs using Acalypha indica and Curcuma longa plants extract.
Experimental procedure
Plant extracts preparation
Acalypha indica and Curcuma longa plants are collected from Herbal garden Coimbatore. For remove soils and dusts, the collected plants are washed in-deionized water followed by underrunning tab water. Thenit’streated shadow drying for 10 days at room temperature. The dried plant materials are crushed to coarsely powdered by grinder and 250 g of plant powder extracted by 250 ml of methanol solvent using soxhlet apparatus28 the prepared plant extracts has been used for further investigation.
Biofabrication of ZnS
The bio-fabrication of ZnS nanoparticles were prepared by using 20 ml plant extract and 1:1 molar ratio of ZnSO4.7H2O (zinc sulfate) and NH2CSNH2(thiourea) as a source materials. The bothZnSO4.7H2O and NH2CSNH2were dissolved in 50 ml of deionized water in two different beakers and the mixtures was stirred 30 min by using magnetic stirrer. While continuous stirring, NH2CSNH2and 20 ml of plant extract solution were added drop by drop into ZnSO4.7H2O solution and the mixture was kept 60 min stirring. The resultant nono-colloidal was formed and it was centrifuged at 2000 rpm for 20 min further it’s heated in air furnace at 1000 C for 12 hours to obtaining fine nanoparticles. Pure ZnS nanoparticles were prepared by same experimental procedure presented above but plant extracts are could not be used for synthesis ZnS, for comparative evaluation prepared ZnS nanoparticles was abbreviated following namely, pure ZnS as ZnS, Acalypha indica and Curcuma longa plant extract as A: ZnS and C: ZnS respectively. Schematic representation for the preparation and antimicrobial activity of Biosynthesized ZnS showed in figure 1.
Figure 1: Schematic representation of pure and bio fabricated ZnS synthesis process.
Characterization method
The Biosynthesized ZnS samples were characterized by XRD technique in the 2θ range of 100-800 with 0.10 step size using Cu-Ka radiation (X’per PRO model-λ = 1.54056 Å) to determine crystal structure. Surface morphology and element compound present in the ZnS was found by scanning electron microscopy with element analyzer (Hitachi S-4500). The optical absorbance and photoluminescence properties were found by Perkin Elmer UV/VIS/ NIR and JASCO FT/1 IR-6600 instrument respectively.
Susceptibility test preparation
Antimicrobial activity of prepared ZnS nanoparticles has been determined by the diameter formation of inhibition zone, In vitro disc diffusion method has been used to investigate the antimicrobial activity of human pathogenic microorganism’s i.e: B.substils, S.aures, E.coli, P.aerugoinosa, C.albicans and A.niger. Microorganisms were maintained 40 C on the slopes nutrient ager, MHA plates and the stock cultures were incubated for 24 hours at 370 C and 250 C respectively. Before transfer culture on the MHA plates, 15 ml of molten media pouring into MHA plates and solidify for 5 min. The minimum amount of stock culture (0.1%) were swapped on the MHA plates surface and 6 mm of sterile disc which has 60 µl concentration was placed on the MHA plate surface. The prepared ZnS samples were loaded on the sterile disc surface and allowed 5 min to diffuse samples into disc, and the loaded MHA plates are kept 24 hours in incubation at 370 C, finally during the incubation inhibition zone was formed around the disc it has been calculated by transparent millimetre ruler.29-30
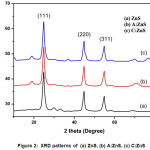
Figure 2: XRD patterns of (a) ZnS, (b) A:ZnS, (c) C:ZnS
Result and Discussion
Structural analysis
XRD pattern of the prepared ZnS, A: ZnS and C: ZnS samples shown in Figure 2. All the prepared samples exhibits three diffraction peaks well matched with zinc blended cubic structure31 From this XRD results shows any other impurity peaks are not detected and its conformed well formation of ZnS NPs. Figure 2 shows theXRD pattern of prepared samples diffraction peaks indicates the presence of prominent peaks 2θ at 28.90, 48.10 and 57.70 corresponding to the miller indices planes to (111), (220) and (311) reflection of ZnS cubic structure which well matched with JCPDS card no.80-0020.32 The XRD pattern of A:ZnS and C:ZnS NPs shows high intensity diffraction peak at (111) plane so it was the evidence bioactive compound of plant extract helps to improve the crystal growth of ZnS nanoparticles. Table 1 shows the calculated average crystalline size of ZnS nanoparticles using Debye Scherer formula
Where, D is the mean crystallite size, β is the full width at half maximum of the diffraction line, θ is diffraction angle, λ is the wavelength of X ray radiation and 0.9 is the constant shape factor.
Table 1: Show the structural properties of pure and bio fabricated ZnS NPs
|
Sample
|
2θ (hkl)
|
FWHM (Radian)
|
D Spacing
|
Average Crystalline size (nm)
|
Band gap energy(eV)
|
|
ZnS
|
28.9 (111)
|
0.4795
|
3.13
|
17.87
|
3.89
|
|
A:ZnS
|
28.9 (111)
|
0.4799
|
3.15
|
17.84
|
3.86
|
|
C:ZnS
|
28.9 (111)
|
0.5056
|
3.28
|
16.94
|
3.82
|
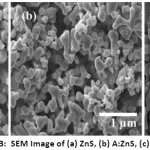
Figure 3: SEM Image of (a) ZnS, (b) A:ZnS, (c) C:ZnS
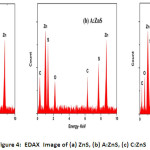
Figure 4: EDAX Image of (a) ZnS, (b) A:ZnS, (c) C:ZnS
Morphological and Element Composition Analysis
The morphological and element composition of prepared ZnS NPs was investigated by using SEM and EDAX. The SEM images of synthesized ZnS NPs are having narrow particle size with spherical shape as shown in Figure 3 (a-c). Surface morphology of the ZnS nanoparticles was varied by the plant extract; from this SEM image revealed bio-fabricated ZnS having a uniform distribution of plant extract and improve the crystalline structure of ZnS NPs as shown in Figure 3 (b-c). The C: ZnS nanoparticle is smaller than ZnS and A: ZnS and diameter of the prepared samples were varied depends on the plant extracts. Figure 4(a-c) shows the EDAX image of pure and biological synthesized ZnS NPs, from this result Zn and S elements were presence in pure ZnS NPs and the Zn (63.59%) composition element was found higher than S (36.41%) element in ZnS NPs and no other impurities observed in the spectrum it was clearly shown in Figure 4 (a). In the EDAX image, A: ZnS NPs composition was confirmed with the presence of Zn, S, Carbon, Oxygen elements in the atomic ratio of 41.23%, 31.94% 16.21% and 10.62% respectively from this spectrum biomolecules of plant extract was influenced to composite the carbon and oxygen element as shown in Figure 4 (b). The Zn, S, Carbon, Oxygen elements also present in C: ZnS in the atomic ratio of 46.24%, 31.93% 15.20% and 06.63% respectively it is shown in Figure 4(c).
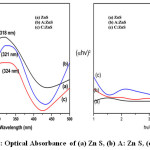
Figure 5: Optical Absorbance of (a) ZnS, (b) A:ZnS, (c) C:ZnS
Optical absorption and Photoluminescence properties
Figure 5 shows the UV-Visible absorbance spectroscopy of prepared samples and all the samples exhibit maximum optical absorbance of 75% was observed. The high absorption peaks at 318 nm, 321 nm and 324 nm for ZnS, A: ZnS and C: ZnS respectively. The bio fabricated ZnS having maximum absorption were found red shifted for ZnS. Tauc’s plot has been used to calculate band gap energy.33
Its relation between absorption coefficient and photon energy and the optical absorption coefficient is α, h is planked constant, A is constant, optical band gap energy is Eg and photon energy is hυ. The calculated band gap value of ZnS, A: ZnS and C: ZnS is 3.89 eV, 3.86 eV and 3.82 eV respectively. From the result band gap was decreased 3.89 (eV) to 3.82(eV) due to the surface roughness of biological synthesized ZnS also the band gap was found higher than bulk ZnS which suitable for cubic structure.
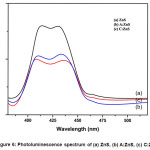
The band gap energy was increased efficiency due to the minimum transition of an electron from the valence band to conductance band. The photoluminescence (PL) spectrum of all the prepared ZnS NPs samples excitation wavelength is 320 nm. The broad peak appearance at 408 nm and 428 nm due to the presence of sulphur and zinc vacancies of all the prepared ZnS samples because PL spectra large intensity depend on the crystalline size and large surface volume ratio of NPs. The Pure ZnS NPs depicts a strong intensity than all other plant extract capped ZnS NPs. C: ZnS NPs intensity was increase due to higher surface volume ratio and smaller particle size of A: ZnS NPs it is shown in Figure 6.
Figure 6: Photoluminescence spectrum of (a) ZnS, (b) A:ZnS, (c) C:ZnS
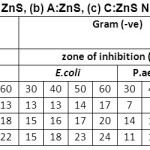
Table 2: Show the ZoI values for (a) ZnS, (b) A:ZnS, (c) C:ZnS NPs against different Microorganisms
Figure 7: Show the ZoI for (a) ZnS, (b) A: ZnS, (c) C: ZnS NPs against different microorganisms
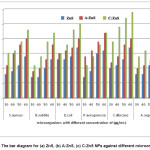
Figure 8: The bar diagram for (a) ZnS, (b) A:ZnS, (c) C:ZnS NPs against different microorganisms
Antimicrobial properties analysis
Antimicrobial activity of prepared ZnS samples has been determined by the formation of inhibition zone, In vitro disc diffusion method has been used to investigate the antimicrobial activity of human pathogenic microorganism’s i.e: B.substils, S.aures, E.coli, P.aerugoinosa, C.albicans and A.niger. Table 2 and figure 7 and 8 shows the zone of inhibition and bar diagram of the inhibition zone against human pathogens. The maximum inhibition zone was observed against E.coli and C.albicans followed by S. aureus, P. aerugoinosa, A. niger and B. subtilis of 17 mm followed by 16 mm, 14 mm, 14 mm, 13 mm at 60 µl of concentration respectively,its clearly indicate when concentration is increased the inhibition zone also increased due high surface absorption of bacteria cell.
For A:ZnS NPs was found highest inhibition zone against P.aerugoinosa followed by E.coli, C.albicans, S.aureus, B.subtilisand A. niger of 22 mm, 20 mm, 20 mm, 14 mm, 18 mm and 15 mm at 60 µl of concentration respectively, P.aerugoinosa has higher inhibition zone at starting concentration when compared to other microorganisms due to presence of bioactive compound of plant extract such as phenolic, saponin, terpenoids, etc. The maximum inhibition zone of C:ZnS NPs was found against C.albicansfollowed by E.coli, S. Aureus, B.subtilis, P.aerugoinosaand A. niger of 29 mm, 25 mm, 23 mm, 22 mm, 22 mm and 20 mm at 60 µl of concentration respectively, from this investigation since at starting concentration C. albicans lead to form high inhibition zone.
From this investigation we found maximum inhibition zone against gram negative bacteria followed by gram positive and fungus culture for bio-fabricated ZnS, this may happen due to presence of bioactive compounds like saponin, phenols, terponoids, proteins and carbohydrates which may help to formation of large surface area nanoparticle penetrate into bacteria cell wall and causes cell death.34-35
Conclusion
In summary, we reported the bio fabricated ZnS NPs were prepared by using plant extracts of Acalypha indica and curcuma longa. The cubic crystalline structure was confirmed for all the ZnS NPs and the average particle was found to be 15-18 nm. The smaller crystalline size was found to bio-fabricated ZnS nanoparticles compare to ZnS NPs and morphology was found the spherical shape like structure. The band gap energy was calculated from 3.89 eV to 3.82 eV and the purity of different element composition of plant extract was reveals from EDAX. The antimicrobial activity was investigated against different human pathogens, maximum zone of inhibition was formed for plant extract mediated ZnS when compared pure ZnS nanoparticles due to influence of smaller crystal size and presence of bimolecular from plant extracts.
Acknowledgements
Authors are thankful to The Lab Head, Thin Film R&D centre, Erode Arts and Science College, Erode for providing lab facilities.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article
Funding Source
The author declares that the funding is done by author only.
Reference
- L. Wang, X. Xu, X. Yuan, Preparation and photoluminescent properties of doped nanoparticles of ZnS by solid-state reaction. J. Lumine. 2010; 130: 137–140.
CrossRef
- M.S. Niasari, N. Mir, F. Davar, Synthesis and characterization of NiO nanoclusters via thermal decomposition. Polyhedron. 2009; 28: 1111-1114.
CrossRef
- M.S. Niasari, F. Davar, Z. Fereshteh, Synthesis and characterization of ZnO nanocrystals from thermolysis of a new precursor. Chemical engineering journal. 2009; 146: 498-502.
CrossRef
- S. Biswas, S. Karc Fabrication of ZnS nanoparticles and nanorods with cubic and hexagonal crystal structures. J. Nanotech. 2008; 19: 45-56.
CrossRef
- M.S. Niasari, M. Reza, L. Estarki, F. Davar, Controllable synthesis of wurtzite ZnS nanorods through a simple hydrothermal method. J. Alloy. Compd. 2009; 475: 782–788.
CrossRef
- M.S. Niasari, M. Ranjbar, D. Ghanbari, A rapid microwave route for the synthesis of ZnS nanoparticles. J. Nanostruct, 2012; 1: 231-235.
- K. Hedayati, A. Zendehnam, F. Hassanpour, Fabrication and characterization of Zinc Sulfide nanoparticles and nanocomposites prepared via a simple chemical preparation method. J. Nanostruct. 2016; 6(3): 207-212.
- M. Sabet, M.S. Niasari, E. Esmaeili, Synthesis of zinc sulfide nanostructures with different sulfur source via mild hydrothermal route: an investigation of crystal phase and morphology. J. Inorg. Organomet. Polym. 2016; 24(4): 738-743.
CrossRef
- F. Davar, M. Mohammadikish, M. Reza, Z. Hamidi, Synthesis of spherical ZnS based nanocrystals using the thioglycolic assisted hydrothermal method, Cryst. Eng. Comm. 2012; 14, 7338-7344.
CrossRef
- J.Z. Liu, P.X. Yan, G.H. Yue, Synthesis of doped ZnS one-dimensional nanostructures via chemical vapour deposition.J. Mate. Letter. 2006; 60: 3471–3476.
CrossRef
- A. Wei, J. Liu, M. Zhuang, Preparation and characterization of ZnS thin films prepared by chemical bath deposition.J. Mater. Sci. Semi. Process. 2013; 16: 1478–1484.
CrossRef
- Devi S. Effect of Mn doping on structural, morphological and optical properties of ZnS nanoparticles by chemical co-precipitation method. J. Appl. Phys., 2014; 6: 06-14.
CrossRef
- S.K. Mani, S. Manickam, V. Muthusamy, R. Thangaraj, Antimicrobial activity and Photocatalytic degradation properties of Zinc Sulfide nanoparticles synthesized by using plant extracts. J Nanostruct, 2018; 8(2): 107-118.
- X.H. Liao, J.J. Zhu, H.Y. Chen, Microwave synthesis of nanocrystalline metal sulfides in formaldehyde solution. J. Mater. Sci. Engin. 2011; 85: 85–89.
CrossRef
- J. Zhu, M. Zhou, M. Xu, Preparation of CdS and ZnS nanoparticles using microwave Irradiation.J. Mater. Letter. 2001; 47: 25–29.
CrossRef
- N.I. Kovtyukhov, E.V. Buzaneva, ZnS and ZnS: Mn films: surface sol-gel synthesis, morphology and photophysical properties. J. Mater. Sci. Engin. 2000; 69: 411–417.
CrossRef
- A. Sobhani, M.S. Niasari, Synthesis, characterization and optical properties of mercury sulfides and zinc sulfides using single-source precursor.J. Mater. Sci. Semic. Proce. 2013; 16: 410–417.
CrossRef
- Q. Zhao, L. Hou, R. Huang, Synthesis of ZnS nanorods by a surfactant-assisted soft chemistry method. J. Inorg. Chem. Comm. 2003; 6: 971-973.
CrossRef
- S. Nizamoglu, T. Ozel, H. Demir, White Light Generation Using CdSe/ZnS Core-Shell Nanocrystals Hybridized with InGaN/GaN Light-Emitting Diodes.J. Nanotech. 2007; 18: 65-70.
CrossRef
- D. Bouhafs, A. Moussi, A. Chikouche, Design and Simulation of Antireflection Coating Systems for Optoelectronic Devices: Application to Silicon Solar Cells.J. Solar Energy Mater Solar Cells. 1998; 52: 79-93.
CrossRef
- P. Wu,H.F. Wang,A Multidimensional Sensing Device for the Discrimination of Proteins Based on Manganese-Doped ZnS Quantum Dots.Chem. Inter. 2011; 50: 8118-8121.
CrossRef
- H. Zhang, X. Chen, Z. Li, Preparation of Sensitized ZnS and its Photocatalytic Activity under Visible Light Irradiation.J. Phys. D Appl. Phys. 2007; 40: 6846-6849.
CrossRef
- G.R. Amir, S Fatahian, N Kianpour. Investigation of ZnS nanoparticle antibacterial effect. Current Nanoscience. 2014; 10: 796–800.
CrossRef
- J. Chen, Binjie Hu, Jinhu Zhi. Optical and photocatalytic properties of Corymbia citriodora leaf extract synthesized ZnS Nanoparticles. Physica E: Low-dimensional Systems and Nanostructures, 2016; 79: 103-106.
CrossRef
- Saeed Mirzadeh, Esmaeel Darezereshki, Fereshteh Bakhtiari. Characterization of zinc sulfide (ZnS)nanoparticles Biosynthesized by Fusarium oxysporum. Materials Science in Semiconductor Processing, 2013; 16: 374–378.
CrossRef
- U. S. Senapati, D. K. Jha, D. Sarkar, Structural, Optical, Thermal and Electrical properties of Fungus guided Biosynthesized Zinc Sulphide Nanoparticles. Research Journal of Chemical Sciences, 2015; 25(1): 33-40.
- U. S. Senapati, D. K. Jha, D. Sarkar, Structural, spectral and electrical properties of green synthesized ZnS nanoparticles using Elaeocarpus floribundas leaf extract. Journal of Materials Science: Materials in Electronics, 2015; 8: 160-168.
CrossRef
- R. Subramanian, P. Subbramaniyan, Double bypasses soxhlet apparatus for extraction of piperine from Piper nigrum.Arab. J. Chem. 2016; 9: 537–540.
CrossRef
- D.T. John, H.J. James, Antimicrobial Susceptibility testing: General Considerations, Manual of Clinical Microbiology 2004; 7th edn. Tamilnadu, India.
- M.K. Lalitha, Manual on Antimicrobial Susceptibility Testing, (2004).
- JCPDS Card Number 80-0020
- J. Tauc, Optical properties and electronic structure of amorphous Ge and Si, J. Mate. Rese. Bull. 1968; 3: 37–46.
CrossRef
- Y. Chen, Q. Ma, H.Y. Wang, Hydrothermal synthesis of ZnS microspheres with highly effective photocatalytic and antibacterial properties. J. Mate. Sci. Mater. Ele. 2016; 27: 10237-10243.
CrossRef
- M. Sathishkumar, Biosynthesis and Characterization of Zinc Sulphide Nanoparticles Using Leaf Extracts of Tridaxprocumbens. Orient. J. Chem. 2016; 33(2): 903-909.}
CrossRef
- G.R. Amir, S. Fatahian, N. Kianpour, Investigation of ZnS nanoparticle antibacterial effect. J. Current Nanosci. 2014; 10: 796–800.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , M. Saroja2 and M. Venkatachalam2
, M. Saroja2 and M. Venkatachalam2
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal