Aeysha Sultan1, Abdul Rauf Raza2, Mian Habib Ur Rehman Mehmood1, Bushra Nisar3, Syeda Laila Rubab1, Ali Irfan3 and Roberto Acevedo4*
1Department of Chemistry, Faculty of Science and Technology, University of Education, Lahore, Pakistan
2Ibn e Sina Block, Department of Chemistry, University of Sargodha, Sargodha-40100, Pakistan
3Department of Chemistry, University of Lahore, Sargodha Campus, Sargodha-40100, Pakistan
4Facultad de Ingeniería y Tecnología. Universidad San Sebastián, Bellavista 7, Santiago 8420524, Chile.
Corresponding Author’s E-Mail: roberto.acevedo.llanos@gmail.com
DOI : http://dx.doi.org/10.13005/msri/160301
Article Publishing History
Article Received on : 26-July-2019
Article Accepted on : 17-Sep-2019
Article Published : 19 Sep 2019
Plagiarism Check: Yes
Reviewed by: EL KACIMI Younes
Second Review by: S KALAISELVAN
Final Approval by: Carvalho Madivate
Article Metrics
ABSTRACT:
The 1-tetralone scaffold and its derivatives are not only important as pharmacological agents but these also serve as precursors for natural products and compounds of medicinal importance. The easiest way to introduce a substituent on an aromatic as well as aliphatic system is nitration. Once introduced, the –NO2 group can be easily replaced by a wide range of functional groups. The review aims to highlight strategies for nitration of substituted and unsubstituted 1-tetralone which led to introduction of NO2 functionality at various positions.
KEYWORDS:
Aliphatic Substitution; Intramolecular Cyclization; Nitration; 1-Tetralone
Copy the following to cite this article:
Sultan A, Raza A. R, Mehmood M. H. R, Nisar B, Rubab S. L, Irfan A, Acevedo R. An Overview of Synthetic Approaches towards of Nitration of α-Tetralones. Mat. Sci. Res. India; 16 (3).
|
Copy the following to cite this URL:
Sultan A, Raza A. R, Mehmood M. H. R, Nisar B, Rubab S. L, Irfan A, Acevedo R. An Overview of Synthetic Approaches towards of Nitration of α-Tetralones. Mat. Sci. Res. India; 16 (3). Available from: https://bit.ly/2mpWfJd
|
Introduction
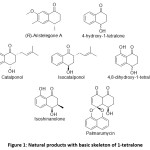
The 1-tetralone 1, a readily available bicyclic ketone with aliphatic as well as aromatic ring, is an economical, inexpensive andvaluable precursor for the construction of a number of natural products and compounds of medicinal importance.1-16 A derivative of 1-tetralone, 4, 7-dimethyl-6-methoxy-1-tetralone, is the fundamental structure of Aristelegone A, a natural product used in Chinese Traditional Medicines.17 4-hydroxy- 1-tetralone is one of the secondary metabolites isolated from Ampelocera edentula. This tetralone derivative has antileishmanial18 and anti diabetic properties.19
4-Hydroxy-1-tetralone is an important structural component of several natural products like catalponol, isocatalponol, isoshinanolone and palmarumycin CP4.20 4,8-dihydroxy-1-tetralone is one of the secondary metabolites isolated from endophytic Aspergilli. This secondary metabolites has been found to exhibit antifungal activity against Alternaria alternata, Alternaria solani, Botrytis cinerea, Candida albicans, Colletotrichum gloeosporioides, Fusarium solani, Fusarium oxysporum f. sp. Niveum, Fusarium oxysporum f. sp. Vasinfectum, Gibberella saubinettii.21
Chalcones derivatives of 1-tetralones have been screened for a wide range of biological activities including anticancer, antifungal, and antibiotic properties.22,23
Figure 1: Natural products with basic skeleton of 1-tetralone
Considering the involvement of 1-tetralone as structural unit of a number of natural products and compounds of medicinal importance, this substrate is of special interest and therefore preparation of its derivatives is of prime importance. Nitration is a very simple and efficient way of bringing a variety of subsituents on aliphatic as well as aromatic system by means of Sand meyer sequence.
Different approaches have been reported for nitration of 1-tetralone that results in introduction of NO2 group at different positions of the tetralone nucleus. For convenience, various strategies concerning nitration of 1-tetralone have been categorized as follows:
1. Direct Nitration of 1-tetralone
1.1. Nitration on aliphatic ring
1.1.1. Nitration on α-carbon
1.1.2. Nitration on C-4
2. Nitration of aromatic nucleus
2.1 Synthesis of 1-tetralones from nitro-precursors
2.2 Oxidation of tetralin
Intramolecular acylation of nitro-substitutedprecursors
Direct Nitration of 1-Tetralone
Nitration on Aliphatic Ring
A number of 2- and/or 4-substituted 1-tetralone derivatives are prevalent in natural as well as in synthetic motifs24-27 however, introducing a substituent at these positions of 1-tetralone is difficult as well as low yielding due to unstable nature of the product as well as the tendency of tetralone to undergo aromatization.28,29
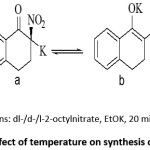
2-nitro-1-tetralones could be synthesized by treating 1-tetralone with dl– as well as d- or l-2-octyl nitrates in presence of potassium ethoxide. The resulting potassium salt was optically inactive.Immediate acid workup resulted in free 2-nitro-1-tetralone which was also optically inactive. The reaction was carried out at 0°C, 22°C and 40°C but temperature appeared to have no effect on optical activity of the product. The authors believe that the reason for the optical inactivity of nitro product was due to existence of potassium salt of 2-nitro-1-tetralone in the form of b.30 This method is of little significance from synthetic point of view since the yield of product was quite low.
Scheme 1: Study of effect of temperature on synthesis of 2-nitro-1-tetralone
The 2-nitro-1-tetralone was synthesized by Feuer et al., from 1-tetralone in reasonable yield by employing alkyl nitrates (R: Et, Pr, Bu, amyl) and potassium alkoxides in presence of non-alcohlic solvents (Et2O, THF, toluene, hexane). The presence of alcohol was found to be detrimental for the product; even small amount of alcohol was reported to result in 2.5-5% reduction of product yield. The reaction worked well at low temperature (-30°C) conditions. For R: amyl in presence of tBuOK, 1-tetralone afforded potassium salt of 2-nitro derivative in 46.2% yield which after acidification to pH of 3.0 resulted in 2-nitro-1-tetralone.31
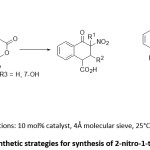
Chiral tetra-substituted 2-nitro-1-tetralone was synthesized by Nath et al., in significant yields via enantioselective Tamura cycloaddition reaction of α-branched nitro-olefins with homophthalic anhydride in the presence of cinchonidine derived squramide as chiral catalyst. Best enantiomeric excess (ee) of 88% was observed with Et2O as solvent. Interestingly, the use of 4Å molecular sieves (MS) was observed to influence the ee of product depending upon the nature of substituent on nitro olefins. With certain substituents, the use ofMS led to an increase of ee while in others the use of the same resulted in significant reduction of ee (scheme 2).32
Scheme 2: Synthetic strategies for synthesis of 2-nitro-1-tetralone
Nitration on C-4
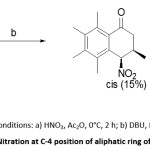
Keumi et al., synthesized polysubstituted 4-nitro-1-tetralones via regioselective nitration of methyl substituted alkenoylbenzenes by using HNO3 and Ac2O. This transformation afforded 2-(nitromethy1)alkenoylbenzenes. The nitromethyl group present ortho to α,β-unsaturated system acts as a Michael donor and undergoesintramolecular Michael addition in presence of a base to afford 4-nitro-1-tetralones (scheme 3).33
Scheme 3: Nitration at C-4 position of aliphatic ring of 1-tetralone
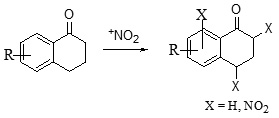
Nitration of Aromatic Nucleus
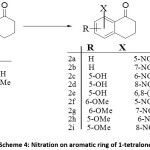
Nitration on aromatic ring is one of the most employed strategies for the functionalization of aromatic systems in synthetic chemistry. A number of strategies and reagents have been employed for nitration of 1-tetralone. In general, it has been observed that direct nitration is often low yielding. The findings of various researchers indicate that the use of alcohol as solvent proves to be detrimental for the nitration product. The reaction works well at low temperature (-30°C) conditions.31 It has also been observed that longer exposure to acid mixture decreases the yield sharply; also effective stirring is important for the reaction, the absence of which leads to formation of side products.34
Scheme 4: Nitration on aromatic ring of 1-tetralone
Ferry et al., carried out successful synthesis of 7-nitro-1-tetralone by utilizing H2SO4 and fuming HNO3 as nitrating mixture. The drop-wise addition of pre-chilled nitrating mixture was carried out over a time period of 20 min at/or below 0°C. Longer addition times and/or prolong acid exposure were observed to result in decreased product yield. After commencement of addition, the reaction was stirred for 20 min and precipitation of product was induced by pouring in ice water. The gummy paste thus formed was allowed to stand overnight during that time the paste hardened. The recrystallization from either afforded pure product with reduced yield of 25% (table 1, entry 1).34
Zhang et al., utilized H2SO4/ HNO3 for nitration of 1-tetralone at -15°C→ ambient. The reaction was completed in 45 minutes and yielded 7-nitro-1-tetralone in 55% yield and the 5-nitro isomer in 26% yield (table 1, entry 2).35
The slow addition of fuming HNO3 to 1-tetralone below 8°C followed by ice treatment of the reaction mixture afforded 7-nitro tetralone as the exclusive product (table 1, entry 3).36
Nitration of 1-tetralone with triflouroacetic anhydride (TFAA) and ammonium nitrate in cooling mixture (comprising of ice/NaCl) afforded 7-nitro in 58% yield. Dichloromethane (DCM)was employed as solvent for the reaction (table 1, entry 4).37
Mahana et al., employed HNO3 in AcOH as nitrating mixture for nitration of 5-hydroxy-1-tetralone. The authors have reported the reaction both at room temperature as well as under refluxing conditions. When the reaction was carried out at room temperature, 6-nitro isomer was isolated as major product in 47% yield while 6,8-nitro-1-tetralone was isolated in 19% yield (table 1, entry 5). the same reaction when carried out under refluxing conditions afforded 6-nitro, 8-nitro and 6,8-dinitro isomers in 21, 48 and 9% yields respectively (table 1, entry 6).38
Ryu et al., synthesized nitro-substituted 5-methoxy-1-tetralones as precursors for transient receptor potential VI (TRPVI) antagonists. The authors reported nitration of 5-methoxy substituted 1-tetralone by employingCu(NO3)2 / Ac2O in Et2O used as a solvent. Reaction was carried out by stirring at room temperature followed by filtration through celite. The reaction yielded 6-nitro and 8-nitro-6-methoxy-1-tetralones in 1:1 yield after flash column chromatography (table 1, entry 7).39
Devkota et al., synthesized nitro derivatives of 6-methoxy-1-tetralones as precursors for water soluble amino acid conjugates. The authors carried out nitration by HNO3 and AcOH in presence ofAc2O used as solvent; the reaction was initially stirred at 0°C for 20 min followed by stirring at ambient temperature for 20 h. Reaction work up and chromatographic purification afforded 5-nitroproduct in 33% yield (table 1, entry 8).40
6-methoxy-5-nitro-1-tetralone and its 7-nitro isomer have the potential to serve as precursors for tubulin binding ligands. These ligands were synthesized by Pinney et al., by carrying out nitration of 6-methoxy-1-tetralone in acetone by stirring to which was added H2SO4/HNO3 at 0°C. The reaction was completed in 6 hours and after workup and column chromatographic purification, the 7-nitro and 5-nitro isomers were isolated in 30 & 35% yields respectively (table 1, entry 9).41
Table 1 summarizes the details of different methodologies for the nitration of unsubstituted and substituted 1-tetralone.
Table 1: Comparison of different strategies for syntheses of nitro-1-tetralone
|
|
Substrate
|
Reagent (eq to substrate)
|
Solvent
|
Time
|
Temp
|
Product (%Yield)
|
|
1
|
1a
|
H2SO4 (4.8), HNO3fuming (2)
|
–
|
45 min
|
0°C
|
2b* (25)34
|
|
2
|
1a
|
H2SO4 (4.4), HNO3 (1.2)
|
–
|
45 min
|
-15-0°C
|
2a (26), 2b (55%)35
|
|
3
|
1a
|
HNO3 fuming (23)
|
–
|
30 min
|
0-8°C
|
2b (major)# 36
|
|
4
|
1a
|
TFAA (1.7), NH4NO3 (1.05)
|
DCM
|
18 h
|
-15-0°C
|
2b (58% approx) 37
|
|
5
|
1b
|
HNO3 (71) /AcOH (28)
|
AcOH
|
45 min
|
reflux
|
2c (21%), 2d (48%), 2e (9%)38
|
|
6
|
1b
|
HNO3 (7.1) /AcOH (28)
|
AcOH/H2O (10:1)
|
45 mn
|
rt
|
2c (47), 2d (19) 38
|
|
7
|
1d
|
Cu(NO3)2 (1) /Ac2O (10.5)
|
Et2O
|
3 h
|
rt
|
2i (46%), 2j (42%) 39
|
|
8
|
1c
|
HNO3 (2), AcOH (1.5), Ac2O (17.5)
|
–
|
21 h
|
0°C – rt
|
2f (33%)*40
|
|
9
|
1c
|
H2SO4 (3.3) / HNO3 (3.5)
|
Acetone
|
6 h
|
0°C
|
2f (35), 2g (30)41
|
*Isolated yield after chromatographic purification; #% yield has not been reported; TFAA: trifloroacetic anhydride
Synthesis of 1-Tetralones from Nitro-Precursors
The direct nitration of substituted and unsubstituted 1-tetralones is associated with low product yields, as evident from table 1. Therefore some alternate attempts have also been reported by a number of researchers that involved indirect preparation of substituted and unsubstituted nitro-1-tetralone.
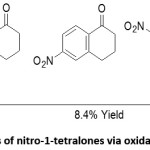
Oxidation of 5-nitrotetralin
Biggs et al., synthesized nitro substituted 1-tetralones as precursors for monoquaternary neuromuscular blocking agents. Nitro-1-tetralones were synthesized from tetralin. Nitration of tetralin yielded 5-nitro and 6-nitrotetralin which upon chromatographic purification afforded 5-nitrotetralin. The CrO3 mediated oxidation of 5-nitrotetralin in presence of AcOH at 70-80C for over 2 hours afforded 5-nitro, 6-nitro 7-nitro and 8-nitro-1-tetralones after methanolic workup and fractional crystallization of the crude product with ligroin in an overall yield of 8.4% (scheme 5).42
Scheme 5: Synthesis of nitro-1-tetralones via oxidation of 5-nitrotetralin
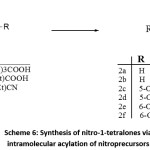
Intramolecular acylation of nitro-precursors
In another method, 7-nitro-1-tetralone was synthesized via intramolecular acylation of p-nitro-γ-phenylbutyric acid in the presence of H3PO4 when heated at 120-125°C in an oil bath for 0.5 hoursusing toluene or anisole as solvent. This protocol leads for the formation of the nitro derivative as minor / side- product. The same protocol gave exceptional yields for p-methoxy- γ-phenylbutyric acid under identical conditions.43
The reaction of 4-(2-nitrobenzene)butyric acid with FSO3H under refluxing conditions afforded 5-nitro and 7-nitro-1-tetralone in an overall yield of 68%. Refluxing 4-(2-nitrobenzene)butanonitrile with FSO3H afforded 5-nitro-1-tetralone isomer as the exclusive product in 68% yield. Another way to obtain 5-nitro-1-tetralone as the exclusive product in good yield (81%) was to reflux 4-(2-nitrobenzene)butyric acid with FSO3H in presence of super acid such as SbF5.44
Scheme 6: Synthesis of nitro-1-tetralones via intramolecular acylation of nitroprecursors
Conclusion
1-tetralone is an important scaffold for a number of chemotherapeutic agents as well as a component of a number of natural products. Nitration of 1-tetralone has been reported by a number of different protocols; each with its own limitations. In this review various strategies for nitration of 1-tetralone have been critically evaluated. It has been observed, that the conditions of nitration vary depending upon the position on which NO2 is desired to be introduced.
In general, the nitration at aliphatic as well as aromatic ring of 1-tetralone, give fruitful results under mild conditions (i.e., low temperature, slow rate of addition of nitrating agent, use of solvent). From the findings of all authors, it is evident that longer reaction time and high temperature conditions result in lower yields. Conversely, instead of using the conventional HNO3/H2SO4 agent, the use of nitrate salts (as a source of nitronium ion) and use of fuming nitric acid afforded products in better yields.
Acknowledgements
The authors of the current article would like to express their gratitude to the following Higher Education Institutions: University of Education, Lahore-Pakistan, University of Sargodha, Sargodha- Pakistan, University of Lahore-Pakistan and Universidad San Sebastian-Chile for their support and encouragement.
Author Contribution
“All authors contributed to obtain the data, to put forward the right questions and to find the replies to them, also to do a proof reading of the manuscript. We have all approved the final version of the manuscript.”
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
References
- J. R. D. McCormick, U. Hirsch, N. O. Sjolander, A. P. Doerschuk, Cosynthesis of tetracyclines by pairs of streptomyces aureofaciens mutants. J. Am. Chem. Soc. 82, 5006 (1960).
CrossRef
- L. P. Mai, F. Gueritte, V. Dumontet, M. V. Tri, B. Hill, O. Thoison, D. Guenard, T. Sevenet, Cytotoxicity of Rhamnosylanthraquinones and Rhamnosylanthrones from Rhamnus nepalensis J. Nat. Prod. 64, 1162 (2001).
CrossRef
- A. A. Hussein, B. Bozzi, M. Correa, T. L. Capson, T. A. Kursar, P. D. Coley, P. N. Solis, M. P. Gupta, Bioactive constituents from three Vismia species. J. Nat. Prod. 66, 858 (2003).
CrossRef
- S. S. Phifer, D. Lee, E. Seo, N. Kim, T. N. Graf, D. J. Kroll, H. A. Navarro, R. A. Izydore, F. Jimenez, R. Garcia, W. C. Rose, C. R. Fairchild, R. Wild, D. D. Soejarto, N. R. Farnsworth, A. D. Kinghorn, N. H. Oberlies, M. E. Wall, M. C. Wani, Antitumor and cytotoxic anthracenone C-glycosides from the leaves of Alvaradoa haitiensis. J. Nat. Prod.70, 954 (2007).
CrossRef
- S. Yao, C. P. Tang, C. Q. Ke, Y. Ye, Abietane Diterpenoids from the Bark of Cryptomeria fortunei. J. Nat. Prod. 71, 1242 (2008).
CrossRef
- M. Fronza, R. Murillo, S. Slusarczyk, M. Adams, M. Hamburger, B. Heinzmann, S. Laufer, I. Merfort, In-vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem.19, 4876 (2011).
CrossRef
- G. Miron-Lopez, I. L. Bazzocchi, I. A. Jimenez-Diaz, L. M. Moujir, R. QuijanoQuiñones, L. Quijano, G. J. Mena-Rejon, Cytotoxic diterpenes from roots of crossopetalum gaumeri, A celastracea species from Yucatan peninsula, Bioorg. Med. Chem. Lett. 24, 2105 (2014).
CrossRef
- L. J. Legoabe, A. Petzer, J. P. Petzer, α-Tetralone derivatives as inhibitors of monoamine oxidase. Bioorg. Med. Chem. Lett., 24, 2758 (2014).
CrossRef
- H. Bayer, C. Batzl, R. W. Hartmann, A. Mannschreck, New aromatase inhibitors. Synthesis and biological activity of pyridyl-substituted tetralone derivatives. J. Med. Chem. 34, 2685 (1991).
CrossRef
- S. Ahmad, L. Doweyko, A. Ashfaq, F. N. Ferrara, S. N. Bisaha, J. B. Schmidt, J. DiMarco, M. L. Conder, T. Jenkins-West, D. E. Normandin, A. D. Russell, M. A. Smith, P. C. Levesque, N. J. Lodge, J. Lloyd, P. D. Stein, K. S. Atwal, Tetrahydronaphthalene-derived amino alcohols and amino ketones as potent and selective inhibitors of the delayed rectifier potassium current IKs. Bioorg. Med. Chem. Lett., 14, 99 (2004).
CrossRef
- J. P. Malerich, T. J. Maimone, G. I. Elliott, D. Trauner, Biomimetic synthesis of antimalarial naphthoquinones. J. Am. Chem. Soc. 127, 6276 (2005).
CrossRef
- C. Fotsch, G. Biddlecome, K. Biswas, J. J. Chen, D. C. D’Amico, R. D. Groneberg, N. B. Han, F. Y. Hsieh, A. Kamassah, G. Kumar, D. Lester-Zeiner, Q. Liu, D. A. Mareska, B. B. Riahi, Y. J. J. Wang, K. Yang, J. Zhan, J. Zhu, E. Johnson, G. Ng, B. C. Askew, A new class of bradykinin 1 receptor antagonists containing the piperidine acetic acid tetralin core. Bioorg. Med. Chem. Lett. 16, 2071 (2006).
CrossRef
- R.-Y. Yang, D. Kizer, H. Wu, E. Volckova, X.-S. Miao, S. M. Ali, M. Tandon, R. E. Savage, T. C. K. Chan, M. A. Ashwell, Synthetic methods for the preparation of ARQ 501 (beta-Lapachone) human blood metabolites. Bioorg. Med. Chem. 16, 5635 (2008).
CrossRef
- M. A. E. P. Mendieta, M. Negri, C. Jagusch, U. Muller-Vieira, T. Lauterbach, R. W. Hartmann, Synthesis, Biological Evaluation, and Molecular Modeling of Abiraterone Analogues: Novel CYP17 Inhibitors for the Treatment of Prostate Cancer. J. Med. Chem. 51, 5009 (2008).
CrossRef
- R. Ortega, H. Hübner, P. Gmeiner, C. F. Masaguer, Aromatic ring functionalization of benzolactam derivatives: new potent dopamine D3 receptor ligands. Bioorg. Med. Chem. Lett. 21, 2670 (2011).
CrossRef
- E. Azuma, N. Nakamura, K. Kuramochi, T. Sasamori, N. Tokitoh, I. Sagami, K. Tsubaki, Exhaustive Syntheses of Naphthofluoresceins and Their Functions. J. Org. Chem. 77, 3492 (2012).
CrossRef
- P. C. Kuo, Y. C. Li, T. S. Wu, Chemical constituents and pharmacology of the Aristolochia species. eJTCM. 2 (4), 249-266 (2012).
- A. Fournet, A. A. Barrios, V. Munoz, R. Hocquemiller, F. Roblot, A. Cave, Antileishmanial Activity of a Tetralone Isolated from Ampelocera edentula, a Bolivian Plant Used as a Treatment for Cutaneous Leishmaniasis. Planta Med. 60, 8–12 (1994).
CrossRef
- T. Y. An, L. H. Hu, R. M. Chen, Z. L. Chen, J. Li, Q. Shen, Anti-diabetes agents – 1. Tetralone derivative from Juglans regia. Chin Chem Lett. 14, 489–90 (2003).
- A. Ghatak, J. M. Dorsey, C. M. Garner, K. G. Pinney, Synthesis of methoxy and hydroxyl containing tetralones: versatile intermediates for the preparation of biologically relevant molecules. Tetrahedron Lett. 44, 4145–8 (2003).
CrossRef
- Z. Huawei, T. Yifei, R. Chuanfen, B. Xuelian, Bioactive Secondary Metabolites from the Endophytic Aspergillus Genus. Rec. Nat. Prod. 10(1), 1-16 (2016).
- M. Z. Gibson, M. A. Nguyen, S. K. Zingales, Design, Synthesis, and Evaluation of (2-(Pyridinyl) methylene)-1-tetralone Chalcones for Anticancer and Antimicrobial Activity. Med Chem. 14 (4), 333-343 (2018).
CrossRef
- J. M. Casey, C. Zhi, P. M. Vani, S. Madhavi, E. S. Tracy, H. Ernest, Z.Heling, L. Ramona, W. Yifan, P.M. Ralph, J. C. David, L. T. Mary, G. P. Kevin, Synthesis of dihydronaphthalene analogues inspired by combretastatin A-4 and their biological evaluation as anticancer agents. Med Chem Comm, 10, 164 (2018).
- K. W. Wang, J. S. Mao, Y. P. Tai, Y. J. Pan, Novel skeleton terpenes from Celastrus hypoleucus with anti-tumor activities. Bioorg. Med. Chem. Lett. 16, 2274-77 (2006).
CrossRef
- J. P. Cueva, A. G. Godoy, J. I. Juncosa, P. A. Vidi, M. A. Lill, V. J. Watts, D. E. Nichols, Probing the Steric Space at the Floor of the D1 Dopamine Receptor Orthosteric Binding Domain: 7α-, 7β-, 8α-, and 8β-Methyl Substituted Dihydrexidine Analogues. J. Med. Chem. 54, 5508-5521 (2011).
CrossRef
- K. P. Devkota, D. Covell, T. Ransom, J. B. McMahon, J. A. Beutler, Growth Inhibition of Human Colon Carcinoma Cells by Sesquiterpenoids and Tetralones of Zygogynum calothyrsum. J. Nat. Prod. 76, 710-714 (2013).
CrossRef
- M. Izawa, S. Kimata, A. Maeda, T. Kawasaki, Y. Hayakawa, Functional analysis of hatomarubigin biosynthesis genes and production of a new hatomarubigin using a heterologous expression system. J. Antibiot. 67, 159-162 (2014).
CrossRef
- A. C. Vargass, I. P. Martin, B. Q. Sire, S. Z. Zard, Synthesis of substituted naphthalenes from α-tetralones generated by a xanthate radical addition–cyclisation sequence. Org. Biomol. Chem. 2, 3018-3025 (2004).
CrossRef
- T. F. Yang, K. Y. Wang, H. W. Li, Y. C. Tseng, T. C. Lien, Synthesis of substituted α-tetralones and substituted 1-naphthols via regioselective ring expansion of 1-acyl-1-indanol skeleton. Tetrahedron Lett. 53, 585-588 (2012).
CrossRef
- W. H. Horne, R. L. Shriner, Asymmetric Syntheses. III. The Action of Optically Active Nitrates on α-Tetralone. J Am. Chem Soc. 55, 4652 (1933).
CrossRef
- H. Feuer, W. Lafayette, J. W. Shephard, Mars, Production of benzonitrocyclic ketones. US Patent 2898376, (1959).
- U. Nath, S. C. Pan, Organocatalytic Asymmetric Tamura Cycloaddition with α-Branched Nitroolefins: Synthesis of Functionalized 1-Tetralones. Org. Chem. 82 (6): 3262–3269 (2017).
CrossRef
- T. Keumi, T. Inagaki, N. Nakayama, M. Taniguchi, T. Morita, H. Kitajima, Ortho-selective side-chain nitration of methyl-substituted alkenoylbenzenes and its application to synthesis of 4-nitro-1-tetralones. J. Org. Chem. 54: 4034-4038 (1989).
CrossRef
- C. W. Ferry, J. S. Buck, R. Baltzly, Org Syn Collective Vol III. 239 (1955).
- H. Adkins, A. G. Rossow, J. E. Carnahan, β-tetralone. J Am Chem Soc. 70 (12), 4247-4248 (1948).
CrossRef
- J. E. Semple, Eur Patent EP0275131, (1988).
- M. Klaus, P. Mohr, US patent US5216148 (1993).
- H. P. Mahana, J. A. Valderrama, Synthesis of 1-Benzazepines as Precursors of 1-Benzazepinediones. Syn Commun. 30 (19), 3481-90 (2000).
CrossRef
- H. C. Ryu, J. O. Lim, D. W. Kang, L. V. Pearce, R, Tran, A. Toth, J. Lee, P. M. Blumberg, Conformationally constrained analogues of N′-(4-tert-butylbenzyl)-N-(4 methylsulfonylamino benzyl) thiourea as TRPV1 antagonists. Eur J med chem, 44, 322-331 (2009).
CrossRef
- L. Davkota, C. M. Lin, T. E. Strecker, Y. Wang, J. K. Tidmore, Z. Chen, R. Guddneooanavr, C. J. Jelinek, R. Lopez, L. Liu, E. Hamel, R. P. Mason, D. J. Chaplin, M. L. Trawick, K. G. Pinney, Bioorg & Med Chem. 24, 938-59 (2016).
CrossRef
- K. G. Pinney, V. P. Mocharia, Z. Chen, C. M. Garner, A. Ghatak, J. M. Dorsey, US patent US 6593374 (2003).
- D. F. Biggs, A. F. Casy, I. Chu, R. T. Coutts, J Med Chem, 19(4), 472-5 (1976).
CrossRef
- S. K. Datta, J Chem Soc, Perkin Trans-1, 62-63 (1972).
CrossRef
- S. Inoki, Y. Motoyama, US 6737548 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.


 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal