Large Scale Synthesis of Nickel Oxide (NiO) by Self Propagated Combustion Reaction
P. Vijayadarshan2, T. Mohan2, J. Venkata Viswanath1, K. J.Venugopal1, N. V. Srinivasa Rao2, Amarnath Gupta2 and A. Venkataraman1, 3*
1Material Chemistry laboratory, Department of Materials Science, Gulbarga University, Kalaburagi-585106, Karnataka. India.
2R and D center, Premier Explosives Limited, P.O. Peddakandukur-508286, Yadadri District, Telangana, India.
3Department of Chemistry, Gulbarga University, Kalaburagi-585106, Karnataka. India.
Correspodning author Email: raman.dms@gmail.com
DOI : http://dx.doi.org/10.13005/msri/140106
Article Publishing History
Article Received on : 25 Dec 2016
Article Accepted on : 25 Jan 2017
Article Published : 03 Mar 2017
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
This is a report on large scale synthesis of nickel oxide (NiO) using polyethylene glycol as a fuel employing self-propagating combustion reaction with nickel oxalate as precursor. The synthesized NiO is characterized for its crystal structure, morphology and bonding via XRD, SEM and FTIR respectively. Thermal behavior of the synthesized NiO is studied employing TGA and DSC. Super paramagnetic behavior of the synthesized is studied by magnetic hysteresis.
KEYWORDS:
Large scale synthesis; NiO; Self-propagating combustion reaction; Super paramagnetic behavior; Thermal studies
Copy the following to cite this article:
Vijayadarshan P, Mohan T, Viswanath J. V, Venugopal K. J, Rao N. V. S, Gupta A, Venkataraman A. Large Scale Synthesis of Nickel Oxide (NiO) by Self Propagated Combustion Reaction. Mat.Sci.Res.India;14(1)
|
Copy the following to cite this URL:
Vijayadarshan P, Mohan T, Viswanath J. V, Venugopal K. J, Rao N. V. S, Gupta A, Venkataraman A. Large Scale Synthesis of Nickel Oxide (NiO) by Self Propagated Combustion Reaction. Mat.Sci.Res.India;14(1). Available from: http://www.materialsciencejournal.org/?p=5343
|
Introduction
The study of transition metal oxides has attracted the attention of materials scientists due to their valuable applications in the areas like optical, electrical, magnetic, mechanical, battery technology or photochemistry, medical fields. The catalytic properties of the metal oxides extend their applicability in propellant manufacturing as burn rate modifiers.1-2 The relation between the structure and properties (both physical and chemical) of metal oxides and their applications are of great importance.3 Metal oxides preparation by the classical solid-state reaction requires a number of stages, including homogenization of the powder precursors, compaction of the reactants, and finally prolonged heat treatment at considerably elevated temperatures.4 Earlier investigators have synthesized NiO nanoparticles adopting various wet chemical synthesis routes.5-6 Current investigation is a self-propagating low temperature combustion route using nickel oxalate precursors for the synthesis of NiO. Polyethylene glycol is used as a fuel for the precursor. In search of a suitable economic fuel, use of polyethylene glycol has given promising results in the conversion of precursor into NiO.7-10
Metal oxide particles generally show evidence for ferromagnetic interaction at low temperatures.9-10 These particles with a uniform size and well dispersion are utilized for many applications such as designing ceramic, magnetic, electro-chromic and heterogeneous catalytic materials.11 Traditionally, synthetic approaches for the production of functional metal oxide materials have involved high-temperature reaction environments with energy – intensive techniques such as laser ablation, ion implantation, chemical vapor deposition (CVD), photolithography or thermal decomposition.12
NiO is a transition metal oxide. Doping of other transition metal elements at Ni site modifies the magnetic, electrical, optical and structural properties to some extent, which opens up the possibility of exploiting this material for various functional applications.13 NiO band structure and electronic properties are subjects of numerous theoretical and experimental studies.14 NiO is considered to be a model semiconductor with p-type conductivity due to its wide band-gap energy range from 3.6 to 4.0 eV.15-16 Most attracting features of NiO are excellent durability and electro chemical stability, low material cost, promising ion storage material in terms of cyclic stability, large span optical density, and possibility of manufacturing by variety of techniques.17 In recent years, NiO nanoparticles has attracted much interest due to its novel optical, electronic, magnetic, thermal, mechanical properties, potential application in catalyst, battery electrodes, gas sensors, electro chemical films, photo electronic devices, and so on as discussed elsewhere. There are many different methods used for the synthesis of NiO which are discussed earlier.17
Experimental techniques for the synthesis of metal oxides are still limited in laboratory scale due to some unresolved problems, such as special conditions, tedious process, complex apparatus, low yield, and high cost.18 From a useful viewpoint, it is vital to develop a way to synthesis high-quality metal oxide powders similar to the one synthesized for γ-Fe2O3 at high through put with low cost.18-19 Current investigation is a self-propagating low temperature combustion route employing metal oxalate precursors and poly ethylene glycol as a fuel.
Experimental
Materials and methods
All the chemicals used are AR grade. Nickel sulphate, oxalic acid and acetone were procured from qualigens fine chemicals. Polyethylene glycol of molecular weight 6,000 grade is obtained commercially (Merck Chemicals). The double distilled water is used throughout synthesis.
Preparations of metal oxalates
Nickel oxalate is synthesized through precipitation reaction in large scale. Two molar solutions of Nickel sulphate and oxalic acid were prepared separately. Nickel sulphate solution is added in the well cleaned large container. Then slowly oxalic acid solution is added in the above solution. By using magnetic stirring at the rate of 1200 rpm and is continued for half an hour more after complete addition of oxalic acid solution. Light Green colored precipitate of nickel oxalate is obtained. The precipitate is filtered and washed with distilled water to remove the excess of ions. Finally washed with acetone to remove the moisture present in the compound and dried under vacuum.
Synthesis of metal oxides
Dried nickel oxalate is grinded to obtain powder and mixed with well ground poly ethylene glycol in 1:5 ratio. Thermal decomposition of nickel oxalate precursor employing a fuel for large scale synthesis NiO is under taken through self-propagating low temperature combustion method. The container is kept in burning chamber and initiated the combustion reaction. The reaction mixture burnt initially with an oxidizing flame which then converts to reducing flame and with a final formation of the residue. The flame once gets ignited is continuous and self-propagating leads to the formation of high surface area NiO as residue. After reaching to room temperature, no traces of carbon impurities were observed in the final residue of metal oxides. As the reaction is fast, i.e. going to completion within 15 min, and ignites auto catalytically, the exact temperature of the reaction could not be measured. The highest temperature on the bases of our earlier studies is understood to be around 5000 C to 6000C.9 Then it is purified by roasting and calcinations.
Characterization techniques
Phase identification of the synthesized metal oxide performed by X-ray diffraction pattern is recorded using Ultima IV Cu/40kv/30mA X-ray diffractometer having K-β filter radiation contains wave length 1.75661 A0. X-ray diffraction pattern of NiO is shown in the figure 1. JEOL JSM-6380LA Scanning Electron Microscopy (SEM) with Energy Dispersive-micro Analysis of X-ray [EDAX] is used to study the particle morphology of NiO samples is shown in figure 2. Magnetic hysteresis is studied by vibrating sample magnetometer (VSM: EG & G Princeton, Applied Research Model 4500) to determine the remanence magnetization (Mr), saturation magnetization (Ms), coercive force (Hc) and remanence ratio (Mr/Ms) of synthesized materials at room temperatures. The Thermo Scientific Nicolet is5 FTIR instrument is used to understand the metal oxygen bonding of the synthesized NiO. Thermal studies are carried out by STA PT – 1000 LINSEIS GERMANY thermal analysis traces were obtained with the atmosphere dynamic dry nitrogen at room temperature to 1000°C, with a heating rate of 10°C /min.
Result and Discussion
The peaks in the figure 1 agreed closely with the data in the [16] shows synthesized NiO sample peaks 2θ = 35.850, 37.460, 43.50, 44.510, 57.530, 63.020, 75.730, 79.520 index as (111), (311), (101), (200), (110) (113), (220), (222). Here 35.850, 37.460, 43.50 peaks are supporting that sample is cubic NiO. Hence all these peaks are indexed to face centered cubic crystalline structure of NiO which is in accordance with the standard spectrum (JCPDS, No. 04-0835, 47-1049).18
Figure 1: XRD of NiO
The presence of the metal and impurities in the synthesized NiO particles were examined by energy dispersive micro analysis of X-ray (EDAX) studies. The EDAX of synthesized NiO is shown in the inset of figure 2. This inset in figure 2 shows the presence of the Ni and O. C and N is with impurity signal. Presence of C and N is evidence for the absorption of moisture from the environment. It was confirmed by FTIR and TGA.
Figure 2 is the Scanning electron micrographs of NiO. The sample has similar morphology, occurrence of particle agglomerates, bulkier is observed. Following observation are made from the SEM images: Figure 2, shows agglomerates of NiO particles due to the moisture content in sample that were absorbed from air. Most of the particles are irregular in shape. The sample having average crystalline size less than 0.1 m small grains are distributed on the surfaces. The particle distribution of small size particles are irregular and are not arisen from nucleation growth of reaction. The well dispersed and single crystal particles of are observed because growth of bigger crystals with less strain among the particles at the time of synthesis. Surface energy and lattice strain play important role for synthesis of metal oxide particles and growing of crystal.
Figure 2: SEM and EDAX of NiO
The presence of the metal and impurities in the synthesized NiO particles were examined by energy dispersive micro analysis of X-ray (EDAX) studies. The EDAX of synthesized NiO is shown in the inset of figure 2. This inset in figure 2 shows the presence of the Ni and O. C and N is with impurity signal. Presence of C and N is evidence for the absorption of moisture from the environment. It is confirmed by FTIR and TGA.
Figure 2 is the Scanning electron micrographs of NiO. The sample has similar morphology, occurrence of particle agglomerates, bulkier is observed. Following observation are made from the SEM images: Figure 2 shows agglomerates of NiO particles due to the moisture content in sample that was absorbed from air. Most of the particles are irregular in shape. The sample having average crystalline size less than 0.1 m small grains are distributed on the surfaces. The particle distribution of small size particles are irregular and are not arisen from nucleation growth of reaction. The well dispersed and single crystal particles of are observed because growth of bigger crystals with less strain among the particles at the time of synthesis. Surface energy and lattice strain play important role for synthesis of metal oxide particles and growing of crystal.
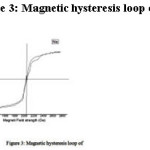
The Magnetic Hysteresis curve for the NiO is shown in figure 3. In this investigation, we have determined the Magnetic hysteresis parameters of saturation magnetization, remanence magnetization and coercively of metal oxides show the magnetic hysteresis traces for NiO and their values are given in table 1. It has no specific saturation magnetization and coercively proves the stronger magnetic induction. The values of these metal oxides were also comparable with that reported for other metal oxides synthesized from the same precursors. The Magnetic Hysteresis loop of metal oxides is evidence for magnetic properties.
Table 1: Hysteresis values of NiO.
|
S.No.
|
Parameters
|
NiO
|
|
1
|
Saturation Magnetization (emu/g)
|
1.86
|
|
2
|
Remanence Magnetization (emu/g)
|
1.85
|
|
3
|
Remanance ratio (Mr/Ms)
|
0.994
|
|
4
|
Coercivity (Hc)
|
113.64
|
|
5
|
+ ve area
|
1.18
|
Figure 3: Magnetic hysteresis loop of NiO
The FTIR spectrum of as synthesized material NiO is shown in figure 4. The NiO shows the absorption region 527.42 cm-1 to 581.03 cm-1 corresponds to the peaks of metal-oxygen vibrational modes. The metal – oxygen frequencies observed for metal oxides in accordance with literature values.19-20 The peaks in between 1361.60 cm-1 to 1028.56 cm-1 is due to the presence of some over tone. The peaks at1601.16 cm-1 to 1615 cm-1 corresponds to H-O-H bonding, the peaks between 3373.68 cm-1 to3325.77 cm-1 corresponds to O-H bonds of water and carbon dioxide present in the air.20-21 This is further supported by TGA.
Figure 4: FTIR of NiO
TGA and DSC studies are shown in figure 5 and 6 respectively. The DSC trace in figure 6 shows the small exothermic peak at 754.235, 823.075, 892.525, 961.825 0C and endothermic peaks at 577.675, 610.225, 685.775, 717.175, 786.775, 855.225, 921.325, 990.075 0C which is assigned to the dehydration of the lattice hydroxyl groups. Heat flow increases with increase in temperature observed upon heating further to 800 0C then remains constant up to1000 0C, which indicates the stability of the dehydrated nickel oxide sample. This higher thermal stability may be due to the effect of the crystallization pathways, which leads to information concerning structural defects. In addition, the amount and the microstructure of the defects also played an important role in the thermal stability of the inorganic materials. The lesser the defect is in the structure, the higher the thermal stability of the crystal. Figure 5 shows the TGA traces of the as synthesized NiO. The TGA shows no peaks and sudden weight loss is observed up to end of the graph i.e. 1000 0C, which indicates the thermal stability of the sample. The total % of weight loss is 13.6 %. The figures 5 show that residual weight loss of NiO is 1.7%. This weight loss is due to the pure magnetic properties of materials. This may be due to the dehydration of the lattice hydroxyl groups strongly bound to the NiO, as is evident from the O-H bands in the IR spectrum. This loss is minimal and therefore the intensity of the O-H band in IR spectrum is weak.
Figure 5: TGA trace of NiO
Figure 6: DSC trace of NiO
Conclusion
Metal oxide is synthesized by self-propagated combustion reaction. The method is cost effective, relatively simple and easy scale up way in a large scale for industrial applications. It is observed from the current study that the particle morphology of the NiO sample is influenced by the precursor. The effect of chemical homogeneity, fine grain structure, particle size and shape of the NiO sample is understood to the electrical properties like electrical conductivity and spectroscopic studies. The present study may find application for magnetic recording, heterogeneous catalyst and sensor for other technological applications. The thermal study (employing TG/DTA techniques) recommends that the phase transition is also controlled by particle morphology. The thermal traces also direct us in understanding the process and possible mechanism of Self-propagating combustion reaction in a generalized way. The exhaustive considerate about the quantitative features and the structural transformations is the upcoming way of number of our works in an industrial scale applications especially as a catalyst in high energy reaction which is in under development.
Acknowledgements
All the authors are thankful to Dr. Amarnath Gupta (CMD, PEL) and Premier Explosives Limited, Hyderabad for financial and technical assistance.
References
- Mc Carroll W. H and Ramanujachary K. V. Oxides Solid-state chemistry, Encyclopedia of Inorganic chemistry, Wiley, USA. 2005;1-47.
- Viswanath J. V., Vijayadarshan P., Mohan T., Srinivasa Rao N. V., Gupta A and Venkataraman A. Copper Chromite as ballistic modifier in a typical solid rocket propellant composition: A novel synthetic route involved. Journal of Energetic Materials. DOI: 10.1080/07370652.2017.1313911.
CrossRef
- Rao C. N. R and Raveau B. Transition Metal Oxides Structure, properties and synthesis of ceramic oxides 2nd edition, Wiley V C H. Germany. 1995.
- Venkataraman A., Vijay A. H., Date S. K and Kulkarni S. D. Bull. Mater. Sci. 2001;24:617–621.
CrossRef
- Nadia N., Zorkipli M., Haida N., Kaus M and Azmin A . M. Procedia Chemistry. 2016;19:626-631.
CrossRef
- El-Kemary M., Nagy N., El-Mehasseb I. Processing. 2013;16(6):1747–1752.
- Sharanabasava V. G., Ravi S. B., Raghunandan D and Venkataraman A. Recent Research in Science and Technology. 2012;4:50-53.
- David R. L. CRC hand book of chemistry and physics, 71st edition. Atomic, molecular and optical physics. FL: CRC press, Boca Raton. 1990-1991.
- Vijay A. H and Venkataraman A. Bull. Mater. Sci. 2003;26:391–396.
CrossRef
- Sharanabasava V. G., Venugopal K. J., Ravi S. B., Srinivasa Rao N. V and Venkataraman A. International Journal of Science Research. 2012;01:77-79.
- Patil K. C., Arona S. T and Ekambarao S. Current Opinion in Solid State Materials. 1997;2:158-165.
CrossRef
- Rao C .N. R. Chemical approaches 10 the Synthesis of inorganic materials, Wiley Eastern Limited, New Delhi. 1994.
- Basavaraja S., Balaji D. S., Mahesh D. B., Raghunandan D., Prithviraj Swamy P. M and Venkataraman A. Bull. Mater. Sci. 2011;34:1313–1317.
CrossRef
- Mallick P and Mishra N. C. American Journal of Materials Science. 2012;2(3):66-71.
CrossRef
- Victor V. V., Wang Z. L and Zou B. S. Chemical Physics Letters. 2001;337:117-124.
CrossRef
- Hao-Long C., Yang-Ming L and Weng-Sing H. Surface & Coatings Technology. 2005;198:138–142.
CrossRef
- Patil P. S and Kadam L. D. Applied Surface Science. 2002;199:211–221.
CrossRef
- Lagashetty A., Havanoor V., Basavaraja S., Balaji S. D and Venkataraman A. Science and Technology of Advanced Materials. 2007;8:484-493.
CrossRef
- Rahman M. M and Venkataraman A. Journal of Thermal Analysis and Calorimetry. 2002;68:91-101.
CrossRef
- Rao C. N. R. chemical applications of infrared spectroscopy. Academic press, New York and London. 1963.
- Gadsden J. A. Infrared spectra of minerals and related inorganic compounds, Butterworths Publications, England. 1975.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal