Synthesis and Study of Microcrystalline Parameters with Electrical Conductivity of Al Doped Lithium Ferrites
Science College, Congress Nagar, Nagpur-12, Maharashtra, India
Corresponding author Email: sureshdarokar@gmail.com
DOI : http://dx.doi.org/10.13005/msri/140216
ABSTRACT:Magnetoplumite type of lithium ferrites substituted by aluminium ions having general chemical formula Li0.5Fe0.5+xAl12-X O19 (where x = 1 to 6) were prepared by standard solid state reaction method The compounds are in single hexagonal phase without traces of uncertainly ambiguous reflection. From XRD pattern lattice parameters has recorded with increasing doped aluminium element in the range from a = 5.807 Å to 5.906 Å and c = 22.507 Å to 22.585 Å pertaining the space group P63/mmc (No.194). The mass density of the ferrites were found linearly varies and depends upon the mass and volume of sample. The X-Ray density has depends upon the lattice constant and molecular weight of the compounds. The average particle size was also estimated. Electrical properties such as dc conductivity, activation energy were studied. Also, the type of carriers responsible in each was determined from thermoelectric studies. The higher resistivity of the compounds Li0.5Fe0.5+xAl12-X O19 suggests less presence of mixed valency cations on equivalent lattice. While Fe show low value due to the ferrous ions present on the octahedral sites along with ferric ions.
KEYWORDS:Activation energy etc; Electrical Conductivity; Lithium ferrite
Introduction
A recent literature survey of magnetoplumbite revealed that the electrical properties of hexagonal1-4 ferrites are less studied compared to its electrical and magnetic properties. This may be due to the fact that they were developed initially as permanent magnet.5-8 Today M-type ferrites are finding applications in many diverse fields like microwave and hence their electrical properties are also becoming important. Many attempts have been made to improve the properties of M ferrites by variety of substitutions. Though M-type ferrite does not contain any monovalent cation, hence a combination of monovalent cation with some multivalent cation for charge compensation may be substituted. With this idea, a new series of materials for permanent magnets of Ba-ferrites were developed for the first time.9-10 Since the properties of ferrite depend on the interactions among the cations distributed over various sites, these were explained on the basis of the knowledge of site distribution of cations in the magnetoplumbite structure. In the present work M-type ferrites having common formula Li0.5Fe0.5+xAl12-X O19 were prepared and their electrical properties such as conductivity, activation energy and thermoelectric power were studied
Materials and Methods
In present investigation the series of M-type compounds, in were prepared using the standard solid-state reaction method.11,12 using AR grade oxides as starting materials. Preparation of ferrites from Hexagonal family are usually difficult as compared to Spinel ferrites and especially the M-type ferrite is one of the most difficult one. This is due to the higher formation temperature and difficulty to get a pure product. The prolonged and continuous heating (1200oC sintering temperature maintained for about 120 hours) used in present work resulted in better product formation as this has given sufficient time to the ions to diffuse. Also the lithium present, in these compounds might have facilitated the synthesis .
In present work, X-ray diffractometer with filtered Cu Kα radiation from X-ray tube, operating at 40 KV and 20 mA were used for identification of compounds. (Philipse PW 1710). From the X-ray graph, formation of hexagonal structure, corresponding to space group P63/mmc or D64h (No 194) was checked.
Two-terminal method was employed for measurement of dc electrical conductivity. The measurements were taken in the range from 150 oC to 700 oC. Finally graphs of ln σ against 1/T were plotted and from the slope of these graphs the activation energies for the compounds were calculated using the relation,
Δ E = 8.617 x 10-5 [ Δ(ln(1/σ) / Δ(1/T ) ]
The Seeback coefficient measurements were carried out using two-probe set up fabricated in the laboratory. The measurements were taken in the temperature range 350oK to 450oK. The type of carriers responsible for conduction was determined in each of the compound from these studies. In order to prepare pellet for the above observations the compound prepared was grounded to fine particle size in an agate mortar. The powder was mixed with 5% polyvinyl acetate solution made in A.R. grade acetone, as binder and mixed thoroughly. This mass was then transferred to a die and pressed under pressure of 5 tons per cm2 using a hydraulic press. The pellets so prepared were then heated in a furnace up to 500 oC to remove the binder. After maintaining this temperature for few hours the pellets were slowly cooled to room temperature. In this way crack free pellets in the shape of a cylinder of small height were obtained. The end faces of the pellets, so prepared, were gently grounded over zero number sand paper to ensure smooth surfaces. The dimensions of the pellets were measured accurately. The smooth and flat parallel faces of the pellets were coated with uniform thin layer of silver paste to facilitate a good electrical contact with the electrodes. The silver paste was dried by heating the pellet slowly for few hours in air at 500 oC. The thin coating of silver paste thus formed was adherent and chemically inert. Pellets were stored in desiccators if found necessary.
Results and Discussion
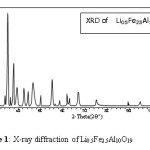
The consolidated data of lattice parameters, Molecular weights and X-ray densities derived from the X-ray diffraction study, for the compounds studied in the present work, are given in table no.1. The formation of predominant M phase in all the compounds was confirmed from the X-ray study (figure No. 1).
Table 1 :Structural data of lithium hexaferrites Consolidated XRD data of Li0.5Fe0.5+xAl12-XO19 (x = 2, 3,4,5& 6)
|
Compounds |
a (A.U.) |
c (A.U.) |
Mol. Wt in gm |
X-ray density gm/cm3 |
|
Li 0. 5 Fe 2. 5 All0O19 |
5.8594 |
22.3234 |
780.89 |
3.9065 |
|
Li 0. 5 Fe 3. 5 Al9O19 |
5.8181 |
21.8796 |
841.79 |
4.3578 |
|
Li 0. 5 Fe 4. 5 Al8O19 |
5.8076 |
21.8411 |
902.66 |
4.6992 |
|
Li 0. 5 Fe 5. 5 Al7O19 |
5.7948 |
21.8077 |
963.54 |
5.0473 |
|
Li 0. 5 Fe 6. 5 Al6O19 |
5.6953 |
21.2626 |
1024.4 |
5.6963 |
X-ray diffraction pattern of Li0.5Fe0.5+xAl12-XO19
Figure 1: X-ray diffraction of Li0.5Fe2.5Al10O19
 Click on image to enlarge |
All the compounds were prepared in polycrystalline form using stoichiometric mixtures of oxides with standard ceramic technique. The phase unicity of the samples were verified by using X-ray diffraction technique. The hexagonal lattice parameters of the compounds were deduced from XRD-pattem. The structural data viz. dobservedand dcalculated and intensity observed with reflection hklindices and the patterns are shown in figure-1. The crystallographic analysis of the samples shows single phase with magnetoplumbite structure. The variation of lattice parameters and cell volume decreases linearly with increase in Al+3 and Fe+3 concentrations. The decrease in lattice parameters and cell volume agree well with the results of Haneda and Kojima[11] for the Ba-ferrites. In M-ferrites, in which the Fe+3 ions occupy five crystallographic sites viz 2a, 2b, 4fi, 4f2 and 12k Lipka et.al. (1990).12 The magnetic behavior of the compounds has been explained, mainly due to the interactions amongst the ions occupying these five sites. Due to several possible site distributions, various comparative magnetic interactions can arise in the lattice and in turn all these decides magnetic properties.
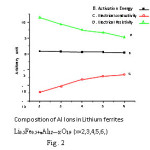
The results of dc electrical conductivity study are shown in the form of plots of conductivity lnσ against inverse absolute temperature. Variation of activation energy, electrical resistivity and electrical conductivity with Al ions in lithium ferrite is shown in figure no. 2. and compiled in the Table No 2. It was observed that the conductivity increases with temperature in all compounds and follows Wilson’s law σ = σo exp(ΔE/kT).
This indicates semi-conducting nature of the compounds. The large values of resistivity and activation energy are attributed to the relatively low number of mixed valency state of an element on an equivalent lattice sites. The increase in conductivity with temperature is attributed to the greater overlap of orbital due to the increased lattice vibrations. Since the overall distances get reduced during overlap, there is a greater probability of exchange of charges between the two overlapping ions. This increased exchange might have led to increase the conduction in the compound.13
Figure 2
 Click on image to enlarge |
The various divalent cations may be categorized as per their atomic radii, masses, magnetic nature and most importantly their site preferences. The exchange interactions responsible for conduction mechanism depends on the overlapping of orbits and hence on actual locations of cations. Therefore the site distribution of these cations on various sites influences the conduction process and hence decides the conductivity of a M-type ferrite. As a result the conduction properties of the compounds prepared in the present work could be explained on the basis of distribution of cations. The electrical properties of the Li0.5Fe0.5+xAl12-X O19 M typeferrite has been explained on the basis of the hopping of electrons in between the ferrous and ferric cations (Fe2+ → Fe3+) present on the octahedral sites. The small quantity of Li, though occupies some of the octahedral sites, there is enough space on these sites for the iron cations. It has been revealed from the Mossbauer and other studies that the divalent Fe2+ cations locate on the octahedral sites of S-block.14-15 Due to this, iron cations of mixed valency on the octahedral sites are developed, which facilitated the electrical conduction. The lowest value of room temperature resistivity for this compound, among all the compounds studied in present work, could be attributed to this phenomenon. It can be that the conductivity value obtained for the compounds in the present investigation is 2.193 x10 ohm-1 cm-1 5.78 x 10 16 ohm-1cm-1. There values of the conductivity may be partly attributed to the low evaporation of lithium from the samples prepared.
Table 2 : Electrical Conductivity and activation energy of Li- Al ferrites
|
Compounds |
Resistivity ρ at room Temp. ohms cm
|
Activation Energy ∆E (ev) |
Conductivity σ at room temp ohms -1 cm -l |
|
Li 0. 5 Fe 2. 5 All0O19 |
4.08 x1011 |
0.73 |
2.447 x 10-11 |
|
Li 0. 5 Fe 3. 5 Al9O19 |
8.21 x108 |
0.60 |
1.218 x 10-11 |
|
Li 0. 5 Fe 4. 5 Al8O19 |
6.21 x108 |
0.57 |
1.631 x 10-9 |
|
Li 0. 5 Fe 5. 5 Al7O19 |
2.38 x 107 |
0.50 |
4.196 x 10-8 |
|
Li 0. 5 Fe 6. 5 Al6O19 |
2.147 x106 |
0.45 |
4.656 x 10-7 |
Conclusion
The formations of compounds were tested by XRD technique. The replacement of Fe +3 ions by Al +3 ions has been investigated because of resemblance of the ionic radii. It is seen that the former ions are very easily replace at any substitution ratio without changing the crystal geometry. The lattice parameter a ans c decrease linearly with substitution ratio in all compounds. The numerical value of the compositional data such as lattice constant, cell volume and X- ray density are reported and same nature in BaM and Sr M- ferrite. The dc electrical conductivity of all the compounds has been calculated from the resistivity measurements from room temperature to 850°K. The consolidated results of the room temperature resistivity and activation energies ∆E(ev) of all the compounds are determined. The resistivity of the compounds show low value due to the ferrous ions present on the octahedral sites along with ferric ions and as a result the conduction takes place in the form of hopping of electron from ferrous to ferric ion. The variation of resistivity in these compounds can be explained on the basis of Verway mechanism.16 Where the conduction is due to the electron exchange between ions Fe+3 and Fe+2 of the same element, present in more than one valence state. The unreacted impurities probably contain same Fe+2 ions which have migrated in growing particles of ferrite (Fe+3 species) subjected to proper sintering of samples. The concentrations of Fe+2 ions so produced is negligibly small which were not detecte.
Aknowledgement
The author is thankful to UGC New Delhi for providing financial assistance to carry out this work under minor research project scheme File No.47-1345/10(WRO). Author also thankful to Dr. N.S. Bhave, Dr. D.K.Burghate, Dr. D.K. Kulkarni, Dr.J.M.Khobragade and Dr.S.B. Khasare to guided and allowed laboratory facilities to work out this series of M-type hexaferrites.
References
- Beretea J and Brown T. Austral.J.Chem.1971;24:273.
- Albanese G., Deriu A., Luchini E and Slokar G. Appli Phys A. 1981;2:45.
CrossRef - Gu B. X., Zang H. Y.,Zhai H.R., Slen B.G., Lu M., Zhang S.Y and Maoi Y. Z. J Phys State Sol. 1992;133:83.
CrossRef - Hanmawalt. International table for X-ray diffraction photograph. 1936.
- Obrador X.,Isalgue A., Collomb A., Tejeda A,Joubert J. C. J.,Phys.C. 1986;19:6605.
- Ghare D. B.,Sinha A. P. B. J.Phys Chem Solid. 1958;29:885.
CrossRef - Laberta A. I., Tejada J., Obradir X. Appl.Phys. 1986;39:221.
- .Darokar S. S., Rewatkar K. G and Kulkarni D. K. Mater Chem.Phys.1998;56:84-85.
CrossRef - Haneda K. Kojima M. J. Appl. Phys 14.B. 1973:376.
- Albense G., Carbulichhio and Deril A. J.Phys Solid State:A. 1974;23:351.
- Haneda K., Kojima H. Phys State Solid (A).1971;6:256.
- .Lipka J., Gruskova A., Orlicky O., Siteck J., Miglierini M.,Grone R. M. Hud and Toth Hyperfine Interaction. 1990;59:381.
CrossRef - Darokar S. S.,Rewatkar K. G & Kulkarni D. K Indian J. Phys. 2000;74(2):155-157.
- Suresh S., Darokar et.al, J. Adv. Appl. Sci. Res. 2013;4(1):173-177.
- Redrigue Z., Obradors X., Labrata A., Tejeja. J., Pernet M. M., Paul S and Tholente J. J. Phys Collog. 1988;149:119.
- Verway E. J., De-Bar. J. H., Trar P. Chem Pay Bull. 1936;55:531.












