Introduction
The aim is to provide a global approach on alternate fuels such as ethanol, biodiesel, vegetable oils, bio-oils, and other bio renewable liquid fuels for the future of energy of transportation. It is possible that sugar cane, Corn, Wood, Straw and even household wastes may be economically converted to bio-fuels like ethanol and biodiesel.
Climatic change is our greatest environmental concern and the scientific consensus is growing politically, need to focus the issue. Not only India across the world, the policies and decision are to be made in this regard. Growing challenges of climate change have shifted focus to the use of cleaner fuels and renewable resources.
Ethanol has emerged as a potential alternative to the fossil sources both as fuel as well as feedstock for various industries. Due to environmental merits, the use of Ethanol in the automotive fuel market will grow fast in the next decade. Ethanol has gained a lot of attention due to green house effect and technological advantages and has the potential to replace fossil fuels like crude oil, etc.; as it contribute significantly to GHG(Greenhouse Gases) emissions saving most of which is imported in India, its use can also yield various other benefits in terms of reducing trade deficit. Most of the ethanol produced in India is using molasses, a byproduct of sugar industry.
Primary consideration involves the production of ethyl alcohol from renewal sources and technical feasibility of using alcohol as an automotive fuel blended with Gasoline. An important reason for interest in renewal energy sources is the concern for Green house effect. Development of Ethanol as a motor fuel can work to fulfill this commitment. Greenhouse Gases (GHG) emission reduction should be closely monitored to save guard an environmental pollution. If ethanol from biomass is used to drive a light-duty vehicle, the CO2 emission is less than 7% of that from the same car using reformulate gasoline. (Bergeron, 1966)
Evaluation of Gasoline-Alcohol Mixtures as Motor Fuel Alternatives
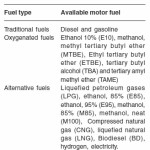
Production of motor fuel alternatives from biomass materials is an important application area of biotechnological methods. Table A shows potential and available motor fuels. Biorenewable sourced motor fuel alternatives are:
1.Gasoline-alcohol mixtures
2.Alcohol substituting gasoline
3.Gasoline – Vegetable oil mixture
4.Diesel Fuel – Vegetable oil mixture
The application of alcohol and gasoline-alcohol mixtures in gasoline (Otto) engines began in the first half of the 20th century. It is possible to find information about various studies on the change in octane numbers of gasoline-alcohol mixtures, composition of the exhaust gases, motor tests, and about the materials used in constructing the engine’s. Between 1980 and 1985 studies intensified on solving the phase separation problem of gasoline-alcohol mixtures.
In gasoline-alcohol mixtures ethanol and methanol are generally used, and in gasoline engine mixtures containing 20% or less alcohol by volume can be used without altering the construction of the engine. Because of the hygroscopic properties of ethanol and methanol the gasoline-alcohol mixtures are in fact ternary mixtures composed of gasoline-alcohol and water. In the evaluation of such mixtures as motor fuel there is the phase separation problem, which depends on several factors. It can be seen in the literatures that there have been numerous attempts to overcome this problem (Mislavskaya et al.,1982; Osten and Sell, 1983).
In gasoline-methanol mixtures containing 0.1% water i-propanol is added to the environment (medium)in order to decrease the phase separation temperature, and Fuels containing different ratios of gasoline-methanol-i-propanol and water are composed, which are proved to be stable in the climatic conditions. An increase in the aromatic character of the gasoline, a decrease in the water content of the mixture, an increase in the amount of the additive used results in a decrease in the phase separation temperature of the mixture. In gasoline- alcohol mixtures various additives like i-propanol, n-butanol, i-butanol, and i-amylalcohol are used.
Evaluation of Vegetable Oils and Diesel Fuel Mixtures as Motor Fuel Alternatives
Since the 1980s important progress has been made on evaluating some low grade oils, oil production wastes, and residues as motor fuel (Pryor et al., 1983). However, direct usage of vegetable oils causes a number of problems concerning the engine because of their high viscosity and the excessive carbonaceous deposits left in the cylinders and on the injector nozzles. Therefore, chemical conversion of vegetable oils was suggested. In order to lower the viscosities and flash points of vegetable oils the transesterifiction method has been applied and it is reported that the alcoholysis products of soybean, sunflower, rapeseed, and used frying oils were proposed as diesel fuel alternatives.
The deregulation of domestic crude oil prices and the formation of OEC have been largely responsible for high fuel prices. The farmer is highly dependent on diesel fuel for crop production. Alternative fuels such as vegetable oils may help easy the petroleum dependence of armers. Recently, the demand for crude oil has decreased because of conservation practices, but ultimately a liquid fuel resource problem exists (Pryor et al., 1983).
Bioalcohols
The alcohols that can be used for motor fuels are methanol (CH3OH), ethanol (C2H5OH), propanol (C3H7OH), and butanol. However, only first two are technically and economically suitable as fuels for internal combustion engines (ICEs), Main commercial bioalcohols from renewable feedstock’s are bioethanol and biomethanol in the worlds energy marketing. Biomethanol currently accounts for more than 94% of global biofuel production, with the majority coming from sugarcane. About 60% of global bioethanol production comes from sugarcane and 40% from other crops. Brazil and united States are the world leaders, which together accounted for about 70% of the world bioethanol production exploiting sugarcane corn respectively. Ethanol was used in Germany and France as early as 1894 by the then incipient industry of internal combustion engines. Brazil has utilized ethanol as fuel since 1925. Currently, ethanol is produced from sugar bets and from molasses. A typical yield is 72.5 liters of ethanol per ton of sugarcane. Modern crops yield 60 tons of sugar cane per hectare of land,. Production of ethanol from biomass is one way to reduce both the consumption of crude oil and environmental pollution. The use of gasohol ( an ethanol and gasoline mixture ) as environmental pollution. The use of gasohol ( an ethanol and gasoline mixture) as an alternative motor fuel has been steadily increasing in the world for a number of reasons. Domestic production and use of ethanol for fuel can decrease dependence on foreign oil, reduce trade deficits, create jobs in rural area, reduce air pollution and reduce global climate change carbon dioxide build up (Bala, 2005).
Alternate Fuels to Gasoline
Gasoline is a blend of hydrocarbons with some contaminants, including sulphur, nitrogen, oxygen and certain metals. The four major constituent groups of gasoline are olefins, aromatics, paraffin’s and naphthenes. The important characteristics of gasoline are density, vapor pressure, distillation range, octane and chemical composition To be attractive, a motor gasoline must have (a) desirable volatility, (b) antiknock resistance(related to octane rating). (c) good fuel economy, (d) minimal deposition on engine component surfaces, and (e) complete combustion and low pollutant emissions (Chigier, 1981).
Alternative fuels for Otto engines or light- duty vehicles (LDVs; cars and light trucks) contain (1) reformulated gasoline, (2) compressed natural gas (3) methanol and ethanol, (4) liquid petroleum gas, (5) liquefied natural gas, (6) Fischer – Tropsch liquids from natural gas, (7) hydrogen and (8) electricity. The electricity can be obtained from (a) spark ignition port injection engines, (b) spark ignition direct injection engines, (c) compression ignition engine, (d) electric motors with battery power,(e) hybrid electric propulsion options, and (f) fuel cells (Demirbas, 2005a).
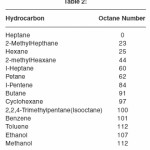
The ,most commonly used measure of a gasoline’s ability to burn without knocking is its octane number. By 1922 a number of compounds had been discovered that were able to increase the octane number of gasoline. Adding as little as 6 ml of tetraethyl lead to a gallon of gasoline, for example can increase the octane number by 15 to 20 units. This discovery gave rise to the first ‘ethyl’ gasoline, and enabled the petroleum industry to produce aviation gasoline’s with octane numbers greater than 100. The octane numbers of common fuels are given in Table 2.
About 10% of the product of the distillation of crude oil is a fraction known as straight-run gasoline burns unevenly in high-compression engine, producing a shock wave that causes the engine to “knock” or “ Ping”. The relationship between knocking and the structure of the hydrocarbons in gasoline is summarized in the following general rules.
a) Branched alkanes and cycloalkanes burn more evenly than straight –chain alkanes.
b) Short alkanes (C6H10) burn more evenly than long alkanes (C7H16)
c) Alkenes burn more evenly than alkanes.
d)Aromatic hydrocarbons burn more evenly than cycloalkanes.
Ethanol Production Process
The hydration of ethylene is the oldest process among the two major ethanol production methods from ethylene (CH2 = CH2) and started more than 100 years ago. The ethanol is prepared from ethylene in a three step process using sulfuric acid (H2SO4). In the first step, the hydrocarbon feedstock containing 35-95% ethylene is exposed to 95-98% sulfuric acid in a column reactor to form monosulfate:
CH2 = CH2 + H2SO4 → CH3CH2OSO3H
It is subsequently hydrolyzed with enough water to give 50-60% aqueous sulfuric acid solution:
CH3CH2OSO3H + H2O → CH3CH2OH + H2SO4
The ethanol is then separated from the dilute sulfuric acid in a stripper column. The last step of this process is to concentrate the sulfuric acid and recycle to the process.In the direct hydration process, an ethylene rich gas is combined with water and passes through a fixed bed catalyst reactor, in which ethanol is formed according to the following reaction (Nelson and Courter, 1954).
CH2 = CH2 + H2O → CH3CH2OH
The ethanol is then recovered in a distillation system.
Ethanol can be obtained from acetylene process in the presence of a proper catalyst such as H2SO4 and HgSO4 ; acetylene/ethyne reacts with water to yield acetaldehyde :
C2H2 + H2O → CH3CHO
Acetaldehyde can be readily reduced by catalytic hydrogenation to ethyl alcohol :
CH3CHO + H2 → CH3CH2OH
The classical catalyst is octacarbonyldicobalt, Co2(CO)8 formed by reaction of metallic cobalt with carbon monoxide.
Grades of ethanol
Ethanol is available in a range of purities that result from its production or, in the case of denatured alcohol, are introduced intentionally.
Denatured Alcohol
Pure ethanol and alcoholic beverage sare heavily taxed as a psychoactive drug, but ethanol has many uses that do not involve consumption by humans. To relieve the tax burden on these uses, most jurisdictions waive the tax when anagenthas been added to the ethanol to render it un fit to drink. These include bittering agents such as denatonium benzoate and toxins such as methanol, naphtha, and pyridine. Products of this kind are called denaturedalcohol.
Absolute alcohol
Absolute or anhydrous alcohol refers to ethanol with alow water content. There are various grades with maximum water contents ranging from 1% to ppm levels. Absolute alcohol is not intended for human consumption. If azeotropic distillation is used to remove water, it will contain trace amounts of the material separation agent (e.g. benzene). Absolute ethanol is used as a solvent for laboratory and industrial applications, where water will react with other chemicals, and as fuel alcohol. Spectroscopic ethanol is an absolute ethanol with a low absorbance in ultraviolet and visible light, fit for use as a solvent in ultraviolet-visible spectroscopy.
Pure ethanol is classed as 200 proof in the USA, equivalent to 175 degrees proof in the UK system.
Rectified Spirits
Rectified spirit, an azeotropic composition of 96% ethanol containing 4%water, is used instead of anhydrous ethanol for various purposes. Wine spirits are about 94% ethanol (188 proof). The impurities are different from those in 95% (190 proof) laboratory ethanol.
Reactions
Ethanol is classified as a primary alcohol, meaning that the carbon its hydroxyl group attaches to has atleast two hydrogen atoms attached to it as well. Many ethanol reactions occur at its hydroxyl group.
Ester Formation
In the presence of acid catalysts, ethanol reacts with carboxylic acids to produce ethylesters and water :
RCOOH + HOCH2CH3 → RCOOCH2CH3 + H2O
This reaction, which is conducted on large scale industrially, requires the removal of the water from the reaction mixture as it is formed. Esters react in the presence of an acid or base to give back the alcohol and a salt. This reaction is known as saponification because it is used in the preparation of soap. Ethanol can also form esters within organic acids. Diethyl sulfate and triethyl phosphate are prepared by treating ethanol with sulfurtrioxide and phosphorus pentoxide respectively. Diethyl sulfate is a useful ethylating agent in organic synthesis. Ethyl nitrite, prepared from the reaction of ethanol with sodium nitrite and sulfuric acid, was formerly a widely used diuretic.
Dehydration
Strong acid desiccants cause the dehydration of ethanol to form diethyl ether and other byproducts. If the dehydration temperature exceeds around 160 °C, ethylene will be the main product. Millions of kilograms of diethyl ether are produced annually using sulfuric acid catalyst:
2CH3CH2OH→CH3CH2OCH2CH3+H2O (on 120 °C)
Combustion
Complete combustion of ethanol forms carbon dioxide and water vapor:
C2H5OH (l) + 3 O2 (g) → 2 CO2 (g) + 3 H2O (g); (ΔHc
= -1371 kJ/mol
specific heat = 2.44 kJ/(kg·K)
Acid-Base Chemistry
Ethanol is a neutral molecule and the pH of a solution of ethanol in water is nearly 7.00. Ethanol can be quantitatively converted to its conjugate base, the ethoxide ion (CH3CH2O–), by reaction with an alkali metal such as sodium:
2 CH3CH2OH + 2 Na → 2 CH3CH2ONa + H2
or a very strong base such as sodium hydride:
CH3CH2OH + NaH → CH3CH2ONa + H2
The acidity of water and ethanol are nearly the same, as indicated by their pKa of 15.7 and 16 respectively. Thus, sodium ethoxide and sodium hydroxide exist in an equilbrium that is closely balanced:
CH3CH2OH + NaOH CH3CH2ONa + H2O
CH3CH2ONa + H2O
Halogenation
Ethanol is not used industrially as a precursor to ethyl halides, but the reactions are illustrative. Ethanol reacts with hydrogen halides to produce ethyl halides such as ethyl chloride and ethyl bromide via an SN2reaction:
CH3CH2OH + HCl → CH3CH2Cl + H2O
These reactions require a catalyst such as zinc chloride. HBr requires refluxing with a sulfuric acid catalyst. Ethyl halides can, in principle, also be produced by treating ethanol with more specialized halogenating agents, such as thionyl chloride or phosphorus tribromide.
CH3CH2OH + SOCl2 → CH3CH2Cl + SO2 + HCl
Upon treatment with halogens in the presence of base, ethanol gives the corresponding haloform (CHX3, where X = Cl, Br, I). This conversion is called the haloform reaction. “An intermediate in the reaction with chlorine is the aldehyde called chloral:
4 Cl2 + CH3CH2OH → CCl3CHO + 5 HCl
Oxidation
Ethanol can be oxidized to acetaldehyde and further oxidized to acetic acid, depending on the reagents and conditions. This oxidation is of no importance industrially, but in the human body, these oxidation reactions are catalyzed by the enzyme liver alcohol dehydrogenase. The oxidation product of ethanol, acetic acid, is a nutrient for humans, being a precursor to acetyl CoA, where the acetyl group can be spent as energy or used for biosynthesis.8
Conclusion
In last 5 years Government of India has taken notable action and put up policies and programs to meet energy requirements and reduce GHG emissions. India has already implemented,Integrated energy policy and is in the process of implementing biofuel policy and has set up an Expert Committee to envisage low carbon economy plan.
Report submitted by Expert Committee on integrated energy policy in 2006 emphasizes, India has been importing ethanol since 2002 and became the largest importer from Brazil in 2005 with imports of 411 million litres. In December 2009 Government of India approved National Policy on Biofuels which targets 20 percent biofuel blending (bio-diesel and bio-ethanol) by 2017. As a comparison, a 5% blend of ethanol in petrol in India would require 1051 million litres of ethanol. Clearly, the foregoing numbers show that domestic availability of ethanol in India (which depends on cane production & yields) and India’s policy on blending alcohol with petrol can significantly affect domestic and world prices of ethanol.
Findings of Expert Committee on integrated energy policy suggest that about half of the domestic ethanol production is used for potable purposes while the balance is used by chemical industry which supplements shortfalls through imports. As per the Expert Committee the value addition in industry is on average 2-3 times higher than the value addition as an additive to petrol. India is the only large country where potable liquor is produced from molasses – most other countries produce potable liquor from grain. For the Indian liquor industry domestic molasses remains the cheapest option since ethanol import for potable purposes attracts a custom duty of 150%. Since the liquor industry provides huge tax revenues to both central and state governments, they have traditionally managed to maintain their hold on domestic molasses based ethanol to meet their need. The remaining domestically available ethanol is simply inadequate to meet the needs of both the domestic chemical industry and blending with petrol. It also points out that Ethanol blending helps diversify energy mix and so improves energy security. If the ethanol is produced domestically the impact on energy security is even better.
Expert committee recommends that let oil companies blend upto 5% of ethanol with petrol but do not mandate oil companies to do so. Expert committee emphasizes that the fate of the chemical industry in India hangs critically in balance between policies that decide end-use of domestically produced ethanol on one hand and the lowering of barriers to international competition in chemicals on the other hand.
With availability of molasses based alcohol from the sugar industry is unlikely to grow significantly, Expert Committee recommendations & findings and implications of national biofuel policy gives rise to an interesting question – whether the optimal use of ethanol is as a fuel, or as a feedstock for producing chemical products. Apart from contributing to reducing GHG emissions, use of ethanol also yield various benefits in terms of reducing import bill by reducing import of oil and chemicals, along with employment generation. A comprehensive socio-economic and environmental analysis need to be considered to acquire a true holistic picture to ascertain optimal use of ethanol.
References
- Alcohol – New World Encyclopedia
- Roach J., Nahmal Geographie News, Retrived 2007-09-03 (2005).
- Alcohol” – 1911 Encyclopedia Britannica
- Couper-As. on a new chemical Theory 1858.
- Faraday M., on new compound of Carbon and Hydrogen and on certain other products obtained during the decomposition of oil by heat” (1825).
- Hennell H., ” on the mutual action of sulphuric acid and alcohol and on the nature of the process by which ether is formed (1825).
- Dipardo, Joseph “Outlook for Biomass Ethanol Production and Demand” “United States Deptt. of Energy Retrived – 2007-09- 22
- Dr.S A Iqbal- “Organic Chemistry Book”

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal