The effluents from mines and metal industries pose serious problems in removal of heavy toxic metal ions. The contents of these metals in effluent are almost above the valid limit1–3; they can be reduced by treatment with lime, but the result is not satisfactory. Thus ion-exchange technique has proved very useful. The ion-exchange resin can be used for metal extraction from ore, analytical reagent, and separation of metal ion and deionization of water.4–10 Most commercial ion- exchange resins are sulfonated polystyrene- divinylbenzene copolymers.11,12 The presence of complex ion-formation in ion-exchange resin has been used to solve the problem.11,12 The aim of the present work is to prepare and study a novel ion- exchange resin.
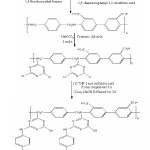
Hence, the present paper comprises the synthesis of novel ion-exchange resin. Hence, the present paper comprises the synthesis of novel ion-exchange resin and its ion-exchanging properties. The synthetic route is shown in Scheme 1 below.
Experimental
Material
All the chemicals used were of either pure or analytical grade.
Synthesis of Polyamines (PA)
The synthesis of polyamine based or 1,4- dichloro methyl benzene and 4,4′- diaminobiphenyl-3,3′-disulfonic acid was performed by a reported method.13 A stoichiometric mixture of 1,4-dichlormethyl benzene, 4,4′- diaminobiphenyl-3,3′-disulfonic acid and NaHCO3 in acetone was refluxed for 3 h. The resultant polymeric product was washed and air-dried and ground to fine powder.
Synthesis of PA-Triazine Resin (PAT Resin)
PA (0.1 mole) was placed in, round-bottom flask containing 100 ml THF, maintained at 50C by external cooling, and stirred. 7.2 gm (0.1 mole) cyanuric chloride was added to this solution and pH was brought to 7–8 by adding NaHCO3. The whole mass was then stirred for 30 min. The product was filtered and was directly used for further reaction.
Synthesis of PAT- Sulfanilic acid (PATS)
To a mixture of PAT product (0.01 mole) and sulfanilic acid (3.7 g, 0.02mole) in THF (100 ml), conc. NaOH was added with maintaining pH 9–10. The mixture was heated to 600C gently for 5 min and was then stirred at room temperature for 8h. The result and gel type material was filtered, washed by water and air-dried. It was powdered to 100 mesh size. Yield was 90%. It did not melt up to 3000C, and was insoluble in water and common organic solvents.
Measurements
The elemental analyses of all PATS samples were estimated by TF-EA-1101 (Italy). The IR spectra were recorded on Nicolet 760 FTIR Spectrophotometer. Sulfanilic acid groups of PATS were determined by a known method14. The batch equilibration method was adopted for the ion- exchanging properties.15,16 The evaluation of the influence of different electrolytes on metal uptake by the polymer, the rate of metal uptake under specified conditions and distribution of various metal ions at different pH values were carried out following the details of the procedures mentioned earlier.15,16
Results and Discussion
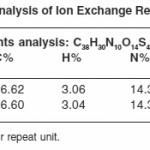
The polymer sample PATS was in the form of a dark brown powder insoluble in common organic solvents. It swelled up to some extent in conc. NaOH solution. It did not melt up to 3000C. The elemental contents in Table 1 are consistent with the predicted structure. The SO3H content of PATS are also in agreement with the structure. The IR spectrum comprises bands due to second NH (3400 cm-1), methylated group (2930, 2850, 1430cm-1), s-triazine and aromatic (3030, 1500, 1600 cm-1).The TGA of PATS contains a single-step degradation. The degradation starts from 2800C, rapidly loss between 300 to 500 and reaches 85% loss at 6500C.
Ion-Exchange Properties
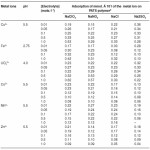
The examination of data presented in Table 2 reveals that the amount of metal ions taken up by a given amount of the PATS polymer depends upon the nature and concentration of the electrolyte present in the solution. The amounts of Fe3+, Cu2+ and UO22+ions taken up by the polymer sample increase with the increase in concentration of ions like chloride, chlorate and nitrate but decrease with the increase in concentration of the sulfate ions. The amounts of the remaining three metal ions, Co2+, Mn2+, and Zn2+, taken by the polymer sample decrease with the increase in concentration of chlorate, chloride, nitrate and sulfate ions.
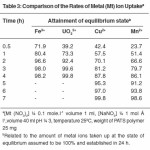
The rates of metal absorption by the PATS sample were measured for Fe3+, UO 2+, Cu2+ and Mn2+ ions in the presence of 1 M NaHCO3 to know the time required to reach the stage of equilibrium. All experiments were carried out at pH 3. An examination of the results presented in Table 3 shows that UO22+ and Fe3+ ions required slightly more than three hours for the establishment of equilibrium and Cu2+ and Mn2+ ions required about five hours for this purpose. In the experiments with solutions containing UO22+ and Fe3+ ions, more than 70% of equilibrium was established in the first hour. This reveals that the rate of uptake of metal ions follows the order UO22+> Fe3+ > Cu2+ > Mn2+. The rates of uptake of Zn2+ and Co2+ ions have been found to be very low at pH 3. Hence the values are not reported.
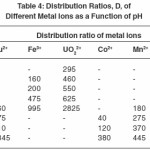
Distribution Ratio of Metal Ions at Different pH Values
The results described in Table 4 reveal that the amount of metal ions taken up by the polymer sample PATS at equilibrium increases with the increase in pH. The selectivity of the polymer sample toward UO22+and Fe3+ ions are higher than towards each of the remaining metal ions. The distribution ratio for Fe3+ ions is lower than that for UO22+ by about 1800 units at pH 3. The lower values of the distribution ratio for Fe3+ ions requires its attachment with proper sites on three different polymer Loci and that of the UO22+ ion requires such an attachment with sites on two polymer Loci. Among the remaining metal ions, Cu2+ has a high value of distribution ratio at pH 6 while the other three metal ions Co2+, Zn2+, and Mn2+ have a low distribution ratio over a pH range from 4 to 6. Further work in the direction of such polymers and their ion exchanging properties is under progress.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal