Nanoparticles with sizes ranging between objects and microparticles have attracted much attention. These particles with various specialized functions not only deepen our understanding of nature, but also serve as the basis for the development of new advanced technology. Nanoparticles are characterized by size-dependent properties both size and surface effects are important. By controlling these, it is possible in principle to design materials of required optical, magnetic, elastic, chemical etc. properties.1 There is increasing interest in the design and synthesis of topological structures composed of monocrystals of various size and shape. Such materials may have unusual optical properties as a result of increasing topological complexity. Inorganic nanoscale building blocks include nanotubes, layered silicates (e.g., montmorillonite, saponite), nanoparticles of metals (e.g., Au, Ag), metal oxides (e.g., TiO2, Al2O3), semiconductors (e.g., PbS, CdS), and so forth, among which SiO2 is viewed as being very important. They have received much attention in recent years and have been employed in a variety of applications in green chemistry. Green chemistry involves then design of chemical syntheses, and the use of material reagents and processes that prevent pollution.
Silicon was first isolated and described as an element in 1824 by a Swedish chemist, Jons Jacob Berzelius. An impure form was obtained in 1811. Crystalline silicon was first produced in 1854 using electrolysis. The reaction between silica and carbon within electric arc furnace produces silicon, the electric arc furnace, was first invented in 1899 by French inventor Paul Louis Toussaint Heroult to make steel. The size, morphology as well as the properties of silica nanoparticles basically depends on the methods of preparation. Hajji et al.,2 nanocomposites systems can be prepared by various synthesis routes, thanks to the ability to combine different ways to introduce each phase an organic part such as a precursor, an oligomer, a preformed linear solution, a network, physically or chemically cross-linked. The mineral part can be introduced as (i) a precursor or (ii) preformed nanoparticle. This leads to a general method for the preparation of silica nanoparticle according to the starting materials and processing techniques: sol-gel processes.
The synthesis, properties, and applications in green chemistry of silica nanoparticle have become a quickly expanding field of research. This paper will give a general overview of the techniques and strategies used for the synthesis of the silica nanoparticle via low temperature method of their characterization, properties, applications in green chemistry, and uses.
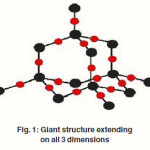
Giant Covalent Structure Of Silicon Dioxide
The chemical compound silicon dioxide, also known as silica, is an oxide of silicon with a chemical formula of SiO2 and has been known for its hardness since antiquity. Silicon dioxide’s exists both as an amorphous and crystalline structures and is frequently found in a three-dimensional polytetrahydral structure where the two oxygen atoms of one SiO2 molecule are associated with a silicon atom of another SiO2 molecules. Crystalline silicon has the same structure as diamond. Tetrahedral arrangement with one silicon surrounded by four oxygen atoms. All silicon atoms are bonded to 4 oxygen atoms. Most oxygen atoms will be bonded to two silicon atoms, so that two tetrahedral are joined at a corner. The orientation can be random, leading to an amorphous structure as shown in figure 1. Then some oxygen atoms are left bonded to only one silicon atom. The relative amount of bridging to non-bridging determines the “quality” of the oxide. If all oxygen atoms are bridging, then a regular crystal structure results– quartz.
Preparation Of Silica Nanoparticle By Sol-Gel Process
Within the past decades, the sol-gel process has been widely used at relatively low temperatures to create novel organic-inorganic composite (hybrid) materials, which were termed “ceramers” by Wikes et al.,3 and “ormosils” or “ormocers” by Schmidt et al.,.4 The development of the field of semiconductor quantum dots through the sol-gel process in the nineties5,6 to enhance the nonlinearity of glass materials, has drawn more attention toward the porosity of sol-gel materials as convenient matrices for the growth of quantum dots. Oxides prepared via this technique exhibit high surface area and have potential for applications such as sorbents, catalysts and electrodes. Many factors influence the kinetics of the hydrolysis and condensation reactions in the sol-gel process, which include the water/silane ratio, catalyst, temperature, the nature of solvent, and so forth. Sol- gel formation process can be simplified to few stages8:
Hydrolysis of the precursor;
Condensation and polymerization of monomers to form the particles;
Growth of particles;
Agglomeration of particles, followed by the formation of networks subsequently, gel structure;
Drying to remove the solvents;
Thermal treatment to remove the surface functional groups and obtain the desired crystal structure. The chemical reaction for synthesis of nano-silica can be summarized as9; there are a number of parameters that affect the process, including pH, temperature, concentration of reagents, H2O/ Si type of catalyst, etc. Sol-gel synthesis is one of the widely used “bottom-up” production methods for silica nanosized materials. The process involves the formation of a colloidal suspension (sol) and gelation of the sol to form a network in a continuous liquid phase (gel). Usually, trimethylethoxysilane or tetraethoxysilane are applied as precursors for synthesis of nanosilica.7 The sol-gel reactions of alkoxysilane can be described as follows:
Hydrolysis
+ROH,R=alkylgroups …(1)
Polycondensation
SiH2O (2)
and/or
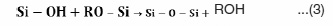
If these sol-gel reactions are complete, full condensed silica is obtained in this process that can be summarized by the following equation:
Si(OR)4+2H2O→SiO2+4ROH …(4)
The most common ceramic precursor is TEOS because it is readily purified and has a relatively slow and controllable rate of reaction.10 When precisely executed, this process is capable of producing perfectly spherical nanoparticles of SiO2 within the size range of 1-100 nm. Processing of natural nanosystems, such as clays, is a promising technique used for manufacturing nanoporous silica materials.7, 11 Silica powder has demonstrated various properties according to its purity, shape, size and distribution and these have been investigated. The sol-gel method has the merit of producing silica, by controlling the size and distribution at low temperatures.
Synthesis Of Silica Nanoparticles
The sol-gel processing of inorganic ceramic and glass materials began as early as the mid 1800s with Ebelman, and Graham’s studies on silica gels.12 The physical properties of nanosized spherical colloidal silica, prepared from tetraethylorthosilicate in ethanol, are difficult to reproduce in that they often depend on the method of isolation of the product. Kolbe in 1956 observed formation of silica particles by reacting TEOS in alkali solution with water in the presence of certain bases. In 1968 Stöber and Fink developed a system of chemical reactions which controlled the growth of spherical silica particles. The details as to “bottom- up” synthesis of nano-SiO2 particles and the effect of this material on the performance of cement systems were reported.13 The nanoparticles of SiO2 were synthesized using sol-gel method, tetraethoxysilane was used as a precursor and the reaction was realized in an acidic or base reaction media with ammonia as catalyst. And, silica gold core-shell particles was synthesized, observed that the plasmon resonance peak position of gold depends upon the shell thickness. Thus, by changing shell thickness, it is possible to design a material with desired optical properties.
Bogush and Zukoski15 obtained monodispersed silica particles having sizes ranging from 40 nm to several micrometers by controlled hydrolysis of tetraethylortho-silicate (TEOS) in ethanol, followed by condensation of the dispersed phase material. Ryu16 had also prepared amorphous silica by oxidation of silicon. Janet et al.,17 produced porous silica gels with high surface areas from tetraethylotho-silicate and polyacrylic acid of high molecular weight in acidic media by sol–gel method. Porous silica glass was prepared through sol-gel processing using tetramethylortho- silicate as precursor, with HNO3 and HF as catalysts.18 Kolbe19 uniform amorphous silica spheres whose sizes ranged from 10 nm to 2 ìm were obtained simply by changing the concentrations of the reactants formed fumed silica is a fine, white, odorless, and tasteless amorphous powder.
And, to synthesis mesoporous silica nanoparticles (MSNs) have attracted more and more attention for their potential biomedical applications and continue research in catalysis, drug delivery and imaging21.The most common types of mesoporous nanoparticles are MCM-41 and SBA-15.20 The mesoporous silica particle could be synthesized using a simple sol-gel method tetraethyl orthosilicate is also used with an additional polymer monomer (as a template). Mesoporous silica nanoparticle (MSN) with tunable physical and surface properties would find application in various biotechnological and biomedical fields.22 In MSN synthesis, various chemical materials, such as sodium silicate, tetramethyl ammonium silicate and tetraethyl orthosilicate etc. are used as silica sources. In efforts to come up with sol-gel methods for the synthesis of MSN with controlled properties, mostly smaller particle sizes, tunable pore diameters, high surface area and high pores volumes show significant advantages over traditional drug nanocarriers. More specifically, the potential utilization of MSN materials in medical and pharmaceutical drug delivery systems is well documented (Courtney et al., 2010; Speybroeck et al., 2010; Vallet-Regi, 2009; Popovici et al., 2010; Leirose and Cardoso, 2011; Limnel et al., 2011; Zhu et al., 2011; Shen et al., 2011; Vivero-Escoto et al., 2010).
It is imperative to have silica particles of a narrow size distribution and a high purity.14 To materials chemistry procedures to synthesize highly efficient SiO2 particles and films controlling nano- level size that can be used for the treatment and disinfection of water and wastewater under even visible light irradiation, and highly sensitive devices for the development of new type of sensors. The synthesis of a wide range of silica nanoparticles with various mean particle sizes will be helpful for modern manufacturers in producing a series of polish grades optimized to a particular application in green chemistry.
Characterization And Properties
The characterization methods used in the analysis of the chemical structure, microstructure and morphology, as well as the physical properties, of the silica nanoparticle are varied. To fully understand structure-property relationships, several characterization techniques are often employed. The properties of the nanoparticle strongly depend on their composition, the size of the particles, interfacial interaction, etc.23
Chemical Properties
Silicon dioxide has covalent bonding and forms a network structure. SiO2 is formed when silicon is exposed to oxygen. A very thin layer (approximately 1 nm or 10 Å) of so-called ‘native oxide’ is formed on the surface when silicon is exposed to air under ambient conditions. Higher temperatures and alternate environments are used to grow well-controlled layers of silicon dioxide on silicon. The amount of atoms residing on the surface increases with the decrease in particle size.24 For silica nanoparticles smaller than 5 nm, more than half of the Si atoms are present on the surface. Thus, the surface should have one or more silanol groups (a≡Si-OH). Therefore, the extent of chemical modification of silica such as grafting of organ functional groups and incorporation of metal ions highly depends on the concentration of silanol groups per grams of silica. The number of silanol groups per unit area of silica provides information regarding the distribution of silanol groups on the silica surface. The concentration of silanol groups increases with the decrease in the particles size which is interrelated to the specific surface area. However, the silanol number decrease with the decrease in the particle size suggests that these nanoparticles could be chemically reactive, therefore suitable for catalyst applications.
Physical Properties
Silica generally behaves as an insulator i.e. it doesn’t show electrical conductivity due to large bandgap energy. There aren’t any delocalized electrons. All the electrons are held tightly between the atoms, and aren’t free to move. It possess very high melting point about 1700oC. It shows very high refractive index and optical absorbance at high temperature. At high temperature, it exists in various crystalline forms as Tridymite, coesite and cristobalite. It has very high dielectric constant, and the physical properties.25 It has a high melting point varying depending on what the particular structure is around 1700°C. Very strong silicon-oxygen covalent bonds have to be broken throughout the structure before melting occurs. It is hard; this is due to the need to break the very strong covalent bonds. It is insoluble in water and organic solvents. There are no possible attractions which could occur between solvent molecules and the silicon or oxygen atoms which could overcome the covalent bonds in the giant structure. Silicon dioxide, a brittle, weak material, has a melting point of 1,728 degrees Celsius. It possesses low thermal conductivity and is therefore a natural thermal insulator.
Mechanical Properties
There are various ways of obtaining mechanical properties data, but the most direct and the least ambiguous in interpretation is testing under uniaxial loading. The determination of the mechanical properties of SiO2 thin films at typical oxidation temperatures and stressed. There is little doubt that time-dependent inelastic deformation plays a significant role in SiO2 films. The study of mechanical behavior of the nanorod entails the proper description of inter-atomic interactions. To developing quantum mechanical methods for treating the inter-atomic forces, and will apply them not only to the nanorod, but also to the effects of water on the strength and deformation of the nanorod. Among the nanoparticles, nano silica was used to improve the properties of cement based materials due to its pozzolanic activity. The excellent mechanical properties and microstructure of cement composite with nano-SiO2 were also reported.26 Li27 reported that incorporating nanoparticles into high- volume fly ash concrete increase significantly the initial pozzolanic activity of fly ash. According to Lin nano- SiO2 particles improve the negative influences of mortar and based on the research of Jo et al.28 nano-SiO2 increases the compressive and flexural strength of concrete and mortar. Silicon dioxide possesses very strong covalent bonding structure and Vander wall’s force of attraction and so this oxide is very strong crack free. It possesses tensile strength about 44 megapascal and modulus of 1005 megapascal. Its impact strength is 7.8 kj/m2.29
Thermal Properties
In many cases of interest to the thermal management of semiconductors, SiO2 is used in the form of a thin layer. Unfortunately, the thermal conductivity of SiO2 is about two orders of magnitude less than that of Si. Hence, even the influence of a very thin layer can be significant. For example, in the case of silicon-on-insulator, thicker oxide layers result in faster devices, but at the cost of a decrease in reliability due to higher temperature. Since the thermal properties of thin films may differ significantly from the bulk value, it is a must to repeat the measurements. A major reason is that the effective TC depends on the way the layer is created and its thickness. Another paper on thermally grown SiO2 showed a clear dependence of the effective TC on the oxide thickness, attributed to an increase in the contribution of the interface.30 Obviously a distinction should be made between intrinsic values and values that include the interfacial resistance between layer and substrate. A final remark on the temperature dependence: the TC shows an increase of about 10% over the range 0-200°C.
Optical Properties
The inter band optical properties of crystalline quartz and amorphous SiO2 vacuum in the ultraviolet UV region have been investigated using combined spectroscopic ellipsometry and UV spectroscopy. Over the range of 1.5–4.2 eV, the optical properties exhibit similar exciton and inter band transitions, with crystalline SiO2 exhibiting larger transition strengths and index of refraction. Crystalline SiO2 has more sharp features in the inter band transition strength spectrum than amorphous SiO2, the energy of the absorption edge for crystalline SiO2 is about 1 eV higher than that for amorphous SiO2. In SiO2, the Si–O–Si bond lengths vary from 1.55 to 1.65 Å while the bond angles vary from 136° to 180° and these changes are found to correlate to changes in the band gap energy from 8.4 to 11 eV.
Philipp observed experimentally the optical properties of crystalline and nanocrstalline SiO2 in the energy range 0–26 eV.31, 32, 33 Loh also measured the optical absorption in fused silica and that of quartz and found them to be very similar.34 Bosio35 and Sorok36 reported the same optical properties of c-SiO2 and a-SiO2. This has been confirmed by many later studies37, 38 leading to the conviction that optical spectra in all SiO2 phases with 4:2 coordination are the same. The complex optical properties of a-SiO2 over the range of 1.5– 42 eV, from which were observed additional inter band transitions at 21.3 and 32 eV 39 and a-SiO2 has similar electronic structure to c-SiO2 over a wide energy range. Optical properties of crystalline and amorphous SiO2 is important because high-purity synthetic SiO2 crystals and glasses are important optical materials, being the basis for optical elements, optical fiber telecommunications, and photolithographic.
Electrical Properties
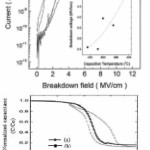
Silicon oxide is an electrically insulating material. Silicon dioxide possess very high band gap energy i.e. the distance between the conduction band and valance band be very large so there be no possibility for the recombination of electrons and hence silicon dioxide can’t shows conduction properties so electrically neutral. An electrical property of the bulk silicon oxide and the SiO2/Si interface by TEOS/O3 was investigated using current- voltage (I–V) and capacitance-voltage (C-V) characteristics were measured by making a metal oxide semiconductor structure with the deposited silicon oxide film.40 From I–V measurements, it was observed that the deposition temperature affected the electrical properties of the bulk oxide, but the TEOS/O3 ratio did not have a strong influence.44 The breakdown strength increased and the leakage current decreased as the deposition temperature was increased.41 43 The breakdown strength was 2.5 and 5 MV/cm at 240 and 380oC, respectively as shown in figure 2(a). The increase of the deposition temperature is thought to make the film structure dense. The C–V curves were obtained at high frequency (1 MHz) in a dark room at room temperature. The result of C–V measurements shows that in figure 2(b), the oxide fixed charge in the films was positive because the C–V curves shifted to the negative direction. The interface trap density for a TEOS/O3 ratio of 0.35 was smallest.
Green Chemistry Applications
Specifically, when this concept is associated with environmental science and engineering to synthesize silica nanostructure functional materials via sol-gel process which are used for environmental remediation, the technology is known with the term “environmental nanotechnology”. Consequently, controlling materials at the nano-level can accelerate the development of new types of products with improved properties and functionalities for environmental applications. Silica metrics is to address the principle of green chemistry. The synthesis mesoporous silica nanoparticles play a different role in the quality of these products is highly dependent on the size and size distribution of the particles. These materials are ubiquitous, and unlikely to react chemically with any other substances in the environment. Moreover, due to their high specific surface area, nanostructures SiO2 particles are also promising as an adsorbent for the removal of toxic trace compounds. The advantages of silica nanoparticles include efficient reinforcement with excellent mechanical strength, heat stability, reduced shrinkage, thermal expansion and residual stress, improved abrasion resistance, and enhanced optical and electric properties.
SiO2 structures exhibiting unique properties such as immiscibility in both water and oil an extremely large surface area and exhibited excellent catalytic activity for the esterification reaction and better yield (with high degree of purity) under mild reaction conditions.45 The mesoporous silica nanoparticles used to inherently possess multiple and simultaneous functions including photo-catalytic decomposition of organic pollutants, destruction of biological toxins, inactivation of pathogenic microorganisms, physical separation of contaminants, host guest chemistry, host for quantum structures46 and anti-biofouling action. These photo-catalytic purification systems can be used as stand-alone technologies or as supplementary and complementary to existing treatment technologies. The amorphous SiO2 nanoparticles are used in fabrication of electronic substrates, thin film substrates, electrical insulators, thermal insulators, humidity sensors.47 The quality of some of these products is highly dependent on the size and size distribution of the silica particles.
Summary And Outlook
This paper aims to prepare and synthesis nano structured SiO2 by low temperature process with structural properties in the form of nanoparticles and films for green chemistry applications. Special attention has been paid to the use of inexpensive precursor and the drying technology to make the production commercial. The sol-gel based MSN synthesis method was established that a delicate balance between all the reagents in the reaction mixture is necessary in order to synthesize nanoparticles with desirable properties and versatile applications. The simple and cheap synthetic logic can be employed to prepare siliceous nanoparticles with different properties. High surface area and porous structure of these materials made them potential candidate to be used for various scientific as well as green chemistry applications. The use of mesoporous materials as catalysts has already been demonstrated in a number of applications, they can be used in separation process, adsorbents, separation of large biological molecules, environmental pollution control etc. These results are the outcome of applying novel concepts of nano-science and nanotechnology, inspired by the potential of this emerging field to provide new directions in the synthesis of advanced catalytic materials with unique hierarchical structures and functionalities. Controlling materials at the nano-level can accelerate the development of new types of products with improved properties and functionalities for green chemistry applications. We hope that this paper will provide some insight knowledge in synthesis of nano silica by sol-gel method, and their used in green chemistry applications.
Acknowledgment
One of the authors (Ruchi Nandanwar) would like to thank the senior research scholars of Department of Physics, M.A.N.I.T, Bhopal (M.P) for helpful discussions.
References
- A. P. Alivisatos, J. Phys. Chem. 100: 13226Chapman & Hall/CRC, (2000)(1996).
- P. Hajji et al, Part B: Poly. Phys. 37: 3172 (1997).
- H. H. Huang, B. Orler, G. L. Wilkes, J. Polym. Bull. 14: 557 (1985).
CrossRef
- H. Schmidt, J. Non-Cryst. Solids 73 (1985) 681
CrossRef
- C. Lin, J. A. Ritter, M. A. Amiridis, J. Non-Cryst. Solids 215: 146 (1997).
CrossRef
- J. M. Miller, L. J. Lakshmi, J. Phys. Chem. B 102: 6465 (1998).
CrossRef
- M. Wilson et al, Nanotechnology–Basic Science and Emerging Technologies, Chapman & Hall/CRC, (2000)
- G. S. Arrard, J. C. Glyde, C. Goltner, Nature 378: 366-368 (1995).
CrossRef
- S. P. Li et al, J. Applied Physics Letters 76: 6 (2000).
- Y. Chen, J. O. Iroh, J. Chem. Mater. 11: 1218 (1999).
CrossRef
- H. J. Fecht, Nature 356 (1992).
- L. L. Hench, J. K. West, Chemistry Reviews 90: 133 (1991).
- K. Sobolev et al, Development of nano-SiO2 based admixtures for high-performance cement-based materials, Progress report, CONACYT, Mexico, (2006)
- M. A. Fardad, E. M.Yeatman, E. J. C. Dawnay, in Proc. SPIE, Sol-Gel Optics III, Edited by J.D. Mackenzie, 77: 2288 (1994)
- G. H. Bogush, C. F. Zukoski, J. Non-Cryst. Solids 104: 95 (1998).
CrossRef
- S. R. Ryu, M. Tomozawa, J. Non-Cryst. Solids352: 3929 (2006) .
CrossRef
- G. Janet, P. G. Galliano, J. Biomaterials 23:4277 (2001).
- A. M. M. Santos et al, J. Non-Cryst. Solids 273: 145 (2000)
CrossRef
- G. Kolbe, Ph.D. Thesis, Friedrich-Schiller- Universität Jena, Germany, (1956)
- A.Yadav et al, J. of Chromatography 1122(1- 2): 13-20 (2006).
- G. Brian et al, J. Chemical Engineering 137:23–29 (2007).
- S. G. Kim, F. Iskandar, K. Okuyama, J. Microporous and Mesoporous Materials 120(3): 447–453 (2009).
CrossRef
- Y. Gao, N. R. Choudhury, In Handbook of Organic-Inorganic Hybrid Materials and Nanocomposites, H. S. Nalwa, Ed., American Scientific Publishers, Stevenson Ranch, CA, 1: 271- 293 (2003).
- K. J. Klabunde, Nanoscale Materials in Chemistry, Wiley-Interscience, New York, USA, (2001)
CrossRef
- S. M. Sze, Physics of Semiconductor Devices, John Wiley and Sons, Inc., New York, (1981)
- Y. Qing et al, J. Const. Build. Mater. 21: 539- 545 (2007).
CrossRef
- G. Li, Cem. Concr. Res. 34: 1043-1049 (2004).
CrossRef
- B. Jo, C. Kim, G. Tae, J. Park, Constr. Build. Mater. 21: 1351-1355 (2007).
CrossRef
- H. P. Fu et al, J. Polym. Adv Technol. 19: 1-8 (2008).
- A. Delan et al, J. Microelectronics Engineering 70: 280-284 (2003).
CrossRef
- H. R. Philipp, J. Solid State Commun. 4: 73 (1966).
CrossRef
- H.R. Philipp, J. Non-Cryst. Solids, 8-10: 627 (1972).
CrossRef
- H.R. Philipp, J. Phys. Chem. Solids 32: 1935 (1971).
CrossRef
- E. Loh, Solid State Commun. 2: 269 (1964).
CrossRef
- C. Bosio, G. Harbeke, W. Czaja, E. Meier, Helv. Phys. Acta: 62: 748 (1989).
- O. M. Sorokin, V. A. Blank, J. Optical Spectroscopy 41: 353 (1973).
- M.Yu. Alexandrov et al, Nuc. Instrum. Methods Phys. Res. A 282: 580 (1989).
CrossRef
- J. E. Rowe, Applied. Physics Letter 25: 576 (1974).
CrossRef
- G. L. Tan, M. F. Lemon, R. H. French, J. Am. Ceramic Soc. 86: 1885 (2003).
CrossRef
- Hyo-Uk Kim, Shi-Woo Rhee, J. Electrochemi. Soc. 147(4): 1473-1476 (2000).
CrossRef
- I. A. Shareef, J. Vac. Sci. Technol. B13: 1988 (1995).
CrossRef
- S. Rojas, L. Zanotti, J. Vac. Sci. Technol. B 11: 2081 (1993).
CrossRef
- D. A. DeCrosta et al, J. Electrochem. Soc. 143: 1079 (1996).
CrossRef
- J. Chou, S. Lee, IEEE Trans. Electron Device,ED-43: 599 (1996).
CrossRef
- A. Rajendran and C. Karthikeyan, J. Amer. Chemi. Science 1(1) (2011) 28-36
- B. Naik and N. N. Ghosh, Recent Patents on Nanotechnology 3: 213-224 (2009).
CrossRef
- P. Sung Kyoo, K. Ki Do, K. Hee Taik, Colloids and Surfaces A: Physicochemical and Engineering Aspects 197: 7–17 (2002)
CrossRef
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal