XPS Characterization of Native Oxide Layers on Fe78B13Si9 Before and After High Energy Heavy Ion Irradiation
Rohit Jain and Shubhra Mathur*
Department of Physics, Jagannath Gupta Institute of Engineering and Technology, Jaipur - 303 905, India.
Article Publishing History
Article Received on : 15 May 2013
Article Accepted on : 25 Jun 2013
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The native oxide layers formed on amorphous alloy Fe78B13Si9 before and after high energy heavy ion (Ni11+ 150 MeV) irradiation, were characterized by X-ray photoelectron spectroscopy (XPS). The unirradiated specimen of the alloy consists of Fe3+ and Fe0 whereas the irradiated specimen contains only Fe3+ species. The B 1s and Si 2p spectra shows the presence of B3+ and Si4+ species most likely in the form of B2O3 and SiO2 on the surfaces of both unirradiated and irradiated specimens of the alloy Fe78B13Si9. The native oxide layer on the irradiated specimen contained higher amounts of oxides of B in comparison to unirradiated specimen.
KEYWORDS:
Amorphous; Irradiation; XPS
Copy the following to cite this article:
Jain R, Mathur S. XPS Characterization of Native Oxide Layers on Fe78B13Si9 Before and After High Energy Heavy Ion Irradiation. Mat.Sci.Res.India;10(1)
|
Copy the following to cite this URL:
Jain R, Mathur S. XPS Characterization of Native Oxide Layers on Fe78B13Si9 Before and After High Energy Heavy Ion Irradiation. Mat.Sci.Res.India;10(1). Available from: http://www.materialsciencejournal.org/?p=316
|
Introduction
Iron based metallic glasses metallic glasses have been the focus of intensive research because of their high magnetic induction, soft magnetic properties and potentially low materials costs.1 Among the ternary Fe-based metal metalloid glasses the Fe78B13Si9 is of particular interest for two reasons (i) it is a parent alloy for many Finemet alloys and close in composition to some commercial metallic glasses targeted for many applications e.g. transformer cores and high frequency applications, and (ii) during crystallization it is possible to study the phase transformation from amorphous to a body centered cubic (b.c.c) (Fe, Si) and a body centered tetragonal Fe2B phase.2-4
The ion beam assisted techniques have made substantial contributions as a selective tool for understanding, fabricating and characterization of amorphous alloys. Ion penetrate a solid and are slowed down (i) by direct transfer of energy to the nuclei by elastic collisions and (ii) by electronic excitation and ionization of atoms by inelastic collisions. The two processes are known as nuclear energy loss Sn and electronic energy loss Se respectively. At high energies (i.e. fast ions), the electronic energy losses are predominant and the density of electron excitations and ionizations becomes so high that new and collective effects arise.5,6 Therefore it would be interesting to examine the effects of high energy heavy ion beam irradiation on the formation of native oxide layers on the surface of amorphous Fe78B13Si9. Change in surface composition of the native oxide layers formed on amorphous Fe73.5B7Si13.5Cu1Nb3 after high energy heavy ion irradiation (Ni11+, 150 MeV) have earlier been reported.7
Analysis of native oxide layers on Fe-B based alloys by XPS has earlier been reported in several investigations8-10 but the effect of ion beam irradiation on Fe-B based alloys are reported in few studies only.7,11,12 The characterization of surface oxide layers is important to understand the role of alloying elements in oxide film formation, especially the metalloids like P, Si and B etc which have been found to affect reactivity and corrosion resistance.10-15 The present paper reports our investigations on characterization of the native oxide layer on Fe-B-Si alloy surface before and after high- energy heavy ion irradiation.
Experimental
Specimen, each of suitable size (1 cm x 1 cm) were cut from amorphous ribbon of Fe78B13Si9, produced by melt-spinning technique and supplied by Vakkumschmelze, Germany. Specimens were cleaned ultrasonically in pure acetone prior to the experiment. For irradiation samples were stuck to the specimen ladder with the help of silver paint. The samples were irradiated with Ni11+ ion beam of 150 MeV energy at a fluence of 1×1014 ions/cm2 at Inter-University Accelerator Centre (IUAC), New Delhi (India).
The surface oxide film on amorphous Fe78B13Si9 alloy was studied by XPS in an electron spectrometer VG Scientific MK II equipped with a non-monochromatized Al (Ka) source (energy ha= 1486.6 eV) and operated at 500 W in a base pressure better than 1.0 x 10-10 m bar in an analysis chamber using hemispherical electron analyzer at a pass energy 50 eV corresponding to an energy resolution of 0.5 eV for Ag 3d5/2 line. A Shirely- type back ground subtraction was performed on the recorded XPS data. The sub-surface layers in the case of native oxide were analyzed by performing sequential sputtering using Ar+ ions of 3.0 keV energy at an ion current of 1 nano-ampere.
Results
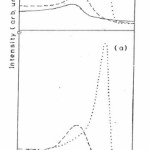
Fig 1 (a) represents the Fe 2p peaks obtained from the native oxide on the as-received surface of the unirradiated specimen and after sputtering with Ar+ ions for 10 and 15 minutes. The Fe 2p3/2 peak occurs at 711.4 eV on the as-received surface and another small peak at 707 eV is also noticed which corresponds to Fe in elemental form. Fig. 1(b) shows the Fe 2p native oxide spectra of the irradiated specimen at the fluence of 1014 ions/ cm2 and after sputtering with Ar+ ions for 10 and 15 minutes. The Fe 2p spectra of as-received irradiated specimen shows only single peak of Fe 2p3/2 at 711.0 eV.14,15
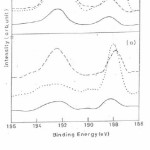
Fig 2 (a) shows B 1s spectrum for unirradiated specimen from the as-received surface and after sputtering with Ar+ ions for 10 and 15 minutes. On the as-received surface B 1s has two peaks appearing at 188.2 eV and 192.3 eV corresponding to boron in pure and oxide form. After sputtering for 10 minutes the oxide appears at 193.0 eV showing a shift to higher binding energy side by about 0.7 eV and after 15 minute sputtering the boron peak at 188.2 eV grows in intensity while the oxide peak at 193.0 eV reduced drastically (Fig 2(a) dotted line curve). Fig 2 (b) shows B 1s spectra for the irradiated specimen at a fluence of 1014 ions/ cm2 and after sputtering with Ar+ ions for 10 and 15 minutes. For the as received surface and specimens after 10 and 15 minute sputtering two peaks appear at 188.2 eV and 192.3 eV corresponding to elemental B and B2O3 oxide but intensities of peaks are larger that of virgin specimen.16
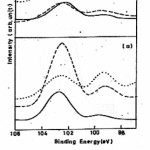
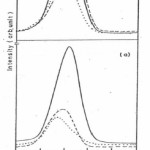
Fig 3 (a) shows the Si 2p3/2 spectra for the as received surface of the virgin alloy and after sputtering with Ar+ ions for 10 and 15 minutes. For the as received surface and specimens after 10 and 15 minute sputtering two peaks appear at 102.7 eV and 99.2 eV corresponding to Si oxide and metallic Si.16,18 After sputtering for 10 minutes again two peaks appear at 99.2 eV and 102.5 eV corresponding to Si in elemental and oxide form. After sputtering for 15 minutes the metal peak grows in intensity and become equal to the intensity of oxide peak. Fig 3 (b) shows Si 2p3/2 spectra for the irradiated specimen and after sputtering with Ar+ ions for 10 and 15 minutes. The presence of Si in the oxide and metallic form was observed and the peak intensities get reduced as comparison to unirradiated specimen.18 Fig 4 (a) and Fig 4 (b) depicts the O 1s spectra from the native oxide on unirradiated and irradiated specimens. The O 1s peak from as-received surface appears at 531.2 eV which may be attributed to the presence of OH–ion on the surface. After sputtering for 10 minutes a broad peak appears at 532.0 eV with clear asymmetry on the high binding energy side 532.7 eV. After sputtering for 15 minutes the O 1s peak appear at 532.8eV and the clear asymmetry at the lower binding energy side 530.8eV. The peak at 532.8eV may be attributed to mixed oxide of boron and silicon16,19 and peak at lower binding energy side may be due to Fe-O bonds. It was also observed that after sputtering on irradiated specimen the intensities of the peaks are much larger as compared to the sputtered surfaces of irradiated specimen. This may lead to that the oxide layer formed on the surface of irradiated specimen is thicker than the virgin one.
Discussion
The Fe 2p spectra (Fig 1(a)) for unirradiated specimen show the presence of Fe3+ species along with some amount of Fe in metallic form. The curve with dashed line after 10 minute sputtering with Ar+ions (Fig. 1a) shows the presence of Fe2+ species at 710.5 eV.16,17 It is known that a partial conversion of Fe3+ to lower valent species might occur due to sputtering effects16 After sputtering for 15 minutes with Ar+ ion the peak corresponding to metallic Fe at 707 eV becomes intense which indicates the increased amount of iron in the elemental form on the surface and the peak representing the oxide component is drastically reduced (Fig 1 (a) dotted curve).
On the other hand the Fe 2p spectra (Fig 1(b)) after irradiation shows only the presence of Fe3+species and no metallic Fe peak is present. After sputtering for 10 minutes the peak at 7110 eV is shifted to 710.5 eV corresponding to a mixture of Fe3+ and Fe2+ species (Fig1 (b) dashed line curve). After further sputtering for 15 minutes (Fig 1(b), dotted line curve) the large single peak occurs at 707.0 eV with a hump at higher binding energy side corresponding to Fe0 and Fe3+/Fe2+ species.
The B 1s spectra for unirradiated specimen shows the presence of pure B and the shift of 4.1 eV of B 1s peak from its position in pure boron pointed to the presence of boron as B3+ species in the oxide film.16 After irradiation the B 1s spectra depicts the presence of B in elemental and oxide form but the intensities of peaks are larger than that of unirradiated specimen. Si 2p spectra of unirrdiated and irradiated specimen (Fig 3(a) and Fig 3 (b)) shows the presence of Si in oxide form most likely in the form of SiO2 as well as Si in metallic form.18 The O 1s spectra (Fig 4 (a) and Fig 4 (b)) for unirradiated and irradiated specimen show the presence of hydroxyl group ions which may arise due to the adsorption of water vapour from the atmosphere.
Therefore it is observed that after irradiation the surface of Fe78B13Si9 alloy is modified and it consist of Fe oxide most likely in the form of Fe3+ and no metallic Fe species, B as B2O3 and Si as SiO2. Hence the results of our study demonstrate the fact that fast ions beams may be employed for surface modification of glassy alloy surface.
Conclusions
X-ray photoelectron spectroscopy revealed the presence of Fe3+, B3+ and Si4+ species after irradiation with Ni11+ ions.
The results of the present study demonstrate the potential of fast ion beams for modification of glassy alloy surface.
Acknowledgements
Thanks are due to M/S Vakkumschmelze, Germany for providing the amorphous alloy ribbon used in this investigation. Assistance of the IUAC staff at Inter-University Accelerator Centre (IUAC), New Delhi is also acknowledged.
References
- Makino A., Kubota T., Makabe M., Chang C.T. and Inoue A., Materials Science Forum 561-565, 1361-1366 (2007).
CrossRef
- Xu Q., Cao X. Z., Sato K., Iwai T. and Yoshiie T., J. Phys. Conf. Ser. 225, 012059 (2010).
CrossRef
- Mat’ko I., Illekovfa E., Svec P. and Duhaj P.,Mater Sci Eng A 225: 145-152 (1997).
CrossRef
- Sato K., Murakami H., Sprengel W., Schaefer H.-E. and Kobayashi Y., Applied Physics Letters 94: 171904 (2009).
CrossRef
- Krasheninnikov A. V. and Nordlund K., Journal of Applied Physics 107: 301-370 (2010).
CrossRef
- Averback R.S.and Bellon P., Appl. Physics 116, 1-28 (2010).
- Jain Rohit, Saxena N. S., Rao K.V.R., AvasthiD. K., Asokan K. and Sharma S. K., Mater. Sci. and Engg. A 297: 105 (2001).
- Arvinda C. L., Bera P., Jayaram V. and Mayanna S. M., Applied Surface Science 191: 128-137 (2002)
CrossRef
- Spolveri I., Atrei A., Bardi U., Torrini M., Gianfranco Rovida and Vasiliev V. Martynyuk. M., Surface Science 419: 303-307 (1999)
CrossRef
- Virtanen S., Moser E.M. and Böhni H., Corrosion Science 36: 373–384 (1994)
CrossRef
- Dubiel S. M., Cieslak J. and Reuther H., Journal of Nuclear Materials 434: 235-239 (2013)
CrossRef
- Miglierini Marcel, Lanèok Adriana and Pavloviè Márius, Proceedings of the International Symposium on the Industrial Applications of the Mössbauer Effect 45-52 (2009).
- Shen Ping, Sun JianXin, Yang Jun, Qi Yan and Chuan Jiang Qi, Nanoscale Research Letters 1-7 (2011)
- Salinas D. R., García S. G., Santo R. Di, Marzo F. F. and Pierna J. B Bessone, A. R., Latin American Applied Research 33: 289-294 (2003)
- López M.R, Escudero M.L., Vida E., Pierna A.R., Marzo F.E and Lorenzo A., Rev. Metal. Madrid 32: 375-380 (1996).
CrossRef
- Wagner C.D., Riggs W.M., Davis L.E., Moulder J.F. and Muilenberg G.E., Handbook of X-ray photoelectron Spectroscopy, Perkin Elmer Corporation, USA, (1979).
- Grosvenor A. P., Kobe B. A., Biesinger M. C. and McIntyre N. S., Surface and Interface Analysis 36: 1564-1574 (2004)
CrossRef
- Suzuki Shigeru, Hasegawa Haijme, Mizoguchi Shozo, Waseda Yoshio, ISIJ INT 43, 71-76 (2003)
CrossRef
- Grosvenor A.P., Kobe B.A. and McIntyre N.S., Surface Science 572: 217-227 (2004).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal