Pramod Kumar1, A. K. Bhargava1, Y. V. S. S. Prasad1, Govind2
1Dept. of Metallurgical and Materials Engineering, MNIT Jaipur, Jaipur (Raj.), India
2Foundry Division, Vikram Sarabhai Space Centre, Trivandrum (Kerala), India
Article Publishing History
Article Received on : 20 Mar 2013
Article Accepted on : 07 May 2013
Article Published : 08 Jan 2014
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this present work, the effect of tin additions was studied on age hardening behaviour of AZ92 magnesium base alloy by hardness and metallographic measurements. For this, three alloys, AZ92, AZ92+ Sn (3%) and AZ92+ Sn (6%) were produced by the sand casting process in the foundry. In tin containing alloys, the tin is added in AZ92 alloys by an amount, 3% and 6% respectively. Samples of these alloys were solution treated at 400ºC for 24 hrs and subsequently aged at 150ºC, 200ºC and 250ºC for various times and ageing plots were obtained by Rockwell hardness measurements. Further Microstructure and XRD analysis were carried out on samples in peak aged condition. The hardness in all these cases increased to peak value.
KEYWORDS:
Sand casting;foundry;heat treatment;precipitation strengthening;XRF;XRD;microstructures;hardness testing
Copy the following to cite this article:
Kumar P, Bhargava A. K, Prasad Y. V. S. S, Govind. Effect of Tin Additions on Age Hardening Behaviour of AZ92 Magnesium Base Alloys. Mat.Sci.Res.India;10(2)
|
Copy the following to cite this URL:
Kumar P, Bhargava A. K, Prasad Y. V. S. S, Govind. Effect of Tin Additions on Age Hardening Behaviour of AZ92 Magnesium Base Alloys. Mat.Sci.Res.India;10(2). Available from: http://www.materialsciencejournal.org/?p=161
|
Introduction
Magnesium base alloy AZ92 has a prime importance in aerospace and automobile industry and has competitive mechanical properties with other magnesium base alloys. Since Age hardening or precipitation hardening is the most commonly used method for strengthening of light alloys1. The fundamental understanding and basis for precipitation hardening, or age hardening, was established in early work at the U. S. Bureau of standards on alloy known as duralumin2.
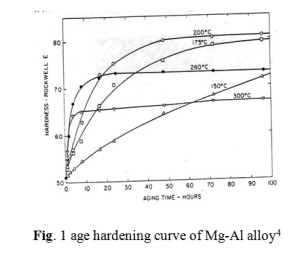
In contrast to the extensive knowledge of precipitation processes and age hardening mechanism in face centered cubic alloys, little is known concerning these processes in hexagonal close packed alloys. In an effort to gain more insight into the mechanism, age hardening studies on a series of magnesium alloys are in progress3. The result of Ageing carried out by Clark4 on Mg-Al alloys at different temperature and time is shown in Fig. 1.
The precipitation process in Mg-Al alloys have been studied5 principally by light micros copy and X-Ray diffraction, within the limitations of these methods, it was determined that precipitation in this system is relatively simple; plates of the equilibrium phases, Mg17Al12, are formed without the intervention of a transition phase. Talbot and Orton6 found that the hardness of maximum was reached when X-Ray evidence showed less than 50% completion of precipitation. A similar result was found by Liontis and Nelson7 in Mg-Al alloys with small amount of zinc.
In Mg-Al alloys both general (intergranular) and cellular (discontinuous) precipitation occur competitively throughout the rage of age hardening temperatures studied by Duly, Simon et.al8. In most cases discontinuous precipitate occurs simultaneously with continuous precipitate. It was also observed by Duly8, et.al. that maximum Vickers hardness corresponds to the end of the precipitation reaction, i.e. the respective volume traction of continuous and discontinuous precipitations does not change for longer ageing times. The decline in hardness is thus probably associated with a coarsening of the structure. According to Talbot6 and Leontis9 maximum hardness is reached when X-ray evidence shows less than 50% completion of precipitation in binary Mg-Al and alloys with a small amount of Zn. Lagowski10 shown in his work that during the precipitation stage, the hardness increases rapidly up to a maximum and then slowly decreases due to over ageing.
The microstructure development of Mg-Al binary alloys and Mg-Al-Zn ternary alloys for a range of ageing temperatures has been investigated in a number of studies10. During ageing of AZ type of alloys the γ-phase precipitates out in two forms: discontinuous and continuous precipitation. Discontinuous precipitation is the cellular growth of alternating layers of γ-phase and near-equilibrium magnesium matrix at high-angle grain boundaries. Growth of the discontinuous precipitation regions ceases relatively early in the precipitation process. Continuous precipitation forms in the remaining regions of the matrix that are not already occupied by discontinuous precipitation. Crawley11 and Lagowski10 have reported that the continuous precipitation consists of relatively large plates on the basal plane of the matrix. A study carried out by Celotto12 for precipitation in AZ alloy by using transmission electron microscope (TEM). According to him no evidence was found in the images or diffraction patterns for precipitation in specimens aged at room temperature for 750 days or in specimens aged at 500C for 260 days.
Experimental Procedure
Specimens Preparation
Casting of specimens in the form of tensile test specimens were carried out by sand casting process in the foundry. For this, three moulds were prepared for the alloys (AZ92, AZ92+Sn (3%) and AZ92+Sn (6%) castings. Further charges as per the composition of alloys were melted in a crucible of 40kg capacity in a pit type induction furnace installed in a foundry. Once the charges are melted it is covered with flux and degassifier. Fluxing was carried out by UE and HE flux and degassing by hexacloroethane tablets. The melts were thoroughly stirred, skimmed and poured into preheated mould with cavities of tensile specimens. The cast tensile specimens were cut from the casting.
Composition Analysis of Casting
Chemical composition analysis of the alloys (AZ92, AZ92+Sn (3%) and AZ92+Sn (6%) alloys) test bars fabricated by sand casting, were studied for knowing the chemical composition by using X Ray Fluorescence (XRF) method. This method is based on fluorescence phenomenon. To carry out chemical analysis of each alloy samples were cut from test bars in the form of diameter 20 mm and thickness of 10 mm. These disc size samples were put into a cavity of XRF machine. Low frequency X rays pass through each sample and fluorescence occurs giving a graph of corresponding elements frequency. These graphs were matched with standard graphs corresponding to different elements.
Heat Treatments
Most of the engineering properties of metals and alloys are related to their microstructure. Equilibrium structure can be predicted for an alloy with the help of an equilibrium phase diagram. The relative proportions of mocroconstituents can change mechanical properties. In practice, change in mechanical properties achieved by a process kwon as heat treatment. This process consists of heating a metal or alloy to a specific predetermined temperature, holding at this temperature for required time and finally cooling from this temperature at predetermined rate. Two type of heat treatment were performed for magnesium alloys
- First solution treatment, for solution treatment samples of each alloy were heated at 400°C in muffle furnace for 24 hrs so as to bring the alloy contents into solution and hot water quenched at 60°C.
- The solution treated Magnesium alloys AZ92, AZ92+Sn (3%) and AZ92+Sn (6%) were aged artificially at three different temperatures 150°C, 200°C and 250°C for 1hr, 5hrs, 10hrs, 15hrs, 20hrs, 25hrs and 30hrs time cycle in Muffle Furnaces under Argon gas protective atmosphere
Hardness Measurements
Rockwell Hardness measurements were carried out in all the three alloys in aged conditions using digital Rockwell hardness tester. The respective load and indenter used were 60kg weight and 1/16“ diameter steel ball. The hardness is expressed in F-scale. For this samples were prepared into disc shape of diameter 15mm and thickness 10 mm approximately and testing carried out according to the ASTM standard.
Metallographic Analysis
The microstructure study of a material can provide the information regarding the morphology and distribution of constituent phases as well as the nature and pattern of certain crystal imperfections. For carrying out microstructural studies of the alloys, first samples were cut into disc shape of diameter 15mm and thickness 10 mm approximately. Secondly Samples were prepared for metallographic examination by conventional method. For sample preparation firstly samples were grinded on grinder for making surface flat. The ground samples were further polished on a series of SiC polishing papers of grades 180, 200, 400 and 600 mounted on a disc-polishing wheel. The intermediate polished samples were fine polished by polishing in suspended alumina particles using disc-polishing machine with velvet cloth on disc. Samples were washed in water and subsequently by methanol and finally dried. The samples were further polished using diamond paste. The diamond polished samples were Ultrasonic cleaned in methanol. The polished samples were etched by picrol acid (5 gm picric acid + 100 ml water) and finally examining the microstructures carried out under PHILIP make optical microscope.
XRD Analysis
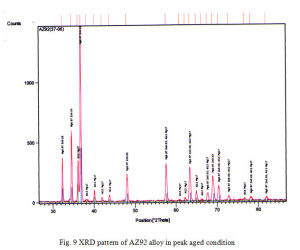
XRD analyses were carried out for AZ92, AZ92+Sn (3%) and AZ92+Sn (6%) alloy in peak aged conditions. For XRD measurements a PHILIPS XRD unit with radiation of Cu Kα (λ=1.54602 Å) was used. Samples of 10 mm diameter and 15 mm thickness were gripped into the holder. The data acquisition was done by computer connected with the XRD machine and the analysis was done by the computer software.
RESULT AND DISCUSSIONS
Compositions Analysis of Sand Casting
The presences of various elements present in the alloys were observed by XRF method. A slight variation in composition is observed from the desired composition. Results of chemical analysis are summarized in table 1. Result shows that the casting is homogeneous.
Hardness Measurement Analysis
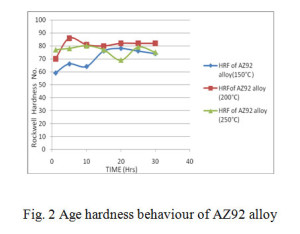
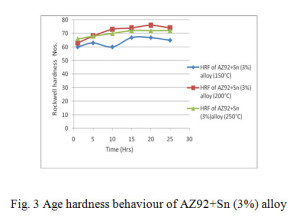
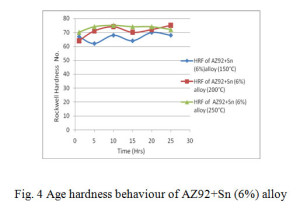
The alloys solutionized at 400°C for 24 hrs and aged at 150°C, 200°C and 250°C over a period of 25 hrs, their Rockwell hardness behaviour curves are shown in figs. 2, 3 and 4 respectively.
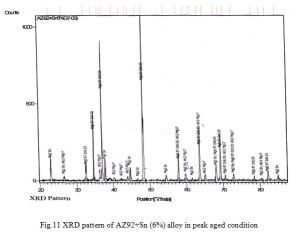
From age hardening curves it is observed that there is one peak in alloy AZ92 and AZ92 with 3% tin. In contrast the alloy contains 6% tin exhibits two hardening peaks. The first hardening peak can be correlated to the precipitation of Mg17Al12. The second peak is expected to arise from the precipitation of Mg2Sn. The formation of Mg2Sn is apparent from the XRD pattern fig. 11. The reflection of Mg2Sn in the XRD pattern of AZ92 with 3% tin is very weak. This explains the absence of second hardening peak in less tin content alloy.
Table 2 shows the peak hardness during ageing at temperatures for 150°C, 200°C and 250°C for various time periods. In all these cases the peak hardness was found at ageing temperature 200°C. However it was found that the peak hardness timings are different
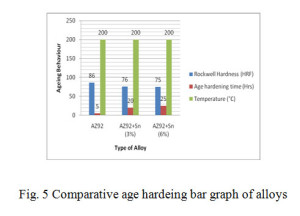
A comparative bar graph shown in Fig.5 shows that obtained hardness remain maximum in AZ92 alloy, the ageing temperature remains constant but ageing time increases in quaternary tin containing alloys.
Metallographic Analysis
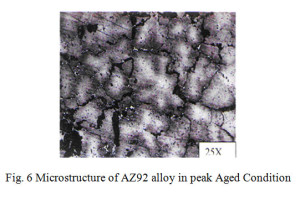
The microstructure of AZ92, as shown in Fig 6, of solution annealed and aged at 200°C for 5 hrs shows continuous and discontinuous precipitate of Mg17Al12.
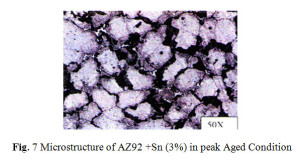
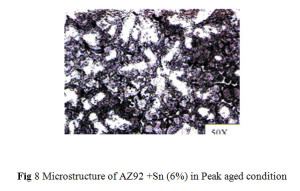
The microstructure of AZ92+Sn (3%) alloy aged at 200°C for 20 hrs is shown in Fig. 7 and aged at 200°C for 25 hrs of AZ92+Sn (6%) in Fig.8 correspondingly. The microstructure of these samples shows precipitate of Mg2Sn. (Lamellar) Mg17Al12 precipitate (light and dark) is observed throughout at the grains of magnesium solid solution after ageing.
XRD Analysis
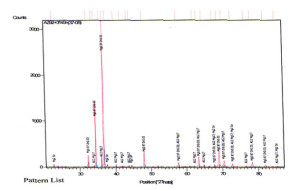
X ray diffraction analysis of AZ92 alloy aged at 150°C reveals magnesium solid solution (α- phase) with precipitate of Mg17Al12 as shown in Fig 9. In case of alloy containing 3% tin the presence of Mg2Sn might not be sufficient in amount to be detected by XRD. The XRD of peak aged hardened AZ92+Sn (3%) and AZ92+Sn (6%) alloy are shown in Fig. 10 and 11. The presence of Mg17Al12 and Mg2Sn phase with the magnesium solid solution can be observed from the XRD patterns.
Conclusions
From this study following conclusions can be drawn.
Chemical analysis carried on sand casting samples shows a homogeneous casting. The introduced chemical compositions for casting of samples are found up to the mark.
From age hardening curves of ternary AZ92 alloy shows one hardness peak which can be correlated to the precipitation of Mg17Al12. The reflection of Mg2Sn in the XRD pattern of AZ92 is very weak. The microstructure shows continuous and discontinuous precipitate of Mg17Al12.
Ageing curves of AZ92+Sn (3%) alloys shows one hardness peak in contrast the alloy contains 6% tin exhibits two hardening peaks. The first hardening peak can be correlated to the precipitation of Mg17Al12l. The second peak is expected to arise from the precipitation of Mg2Sn. The formation of Mg2Sn is apparent from the XRD pattern of 6 % tin containing AZ92 alloy. The reflection of Mg2Sn in the XRD pattern of AZ92 with 3% tin is very weak. This explains the absence of second hardening peak in less tin content alloy. The microstructure of these samples shows precipitate of Mg2Sn. (Lamellar) Mg17Al12 precipitate (light and dark) is observed throughout at the grains of magnesium solid solution after ageing.
The peak hardness of tin contain alloys is less than of AZ92 alloys. This is due to suppression of hard phase Mg17Al12. X-ray analysis do not show Mg17Al12 traces in 6% tin containing alloy. The amount of this phase formation reduced in 3% tin containing alloy compared to AZ92 alloy.
Acknowledgement
Present work was carried out by the author during his post graduate study in metallurgy from the MNIT Jaipur. Author is highly thankful to his supervisors for their valuable support and guidance throughout the project work. Author is highly thankful to M/s VSSC Trivandrum for providing opportunity to work in their labs
References
- Cahn J. W., Bull. Alloy Phase Diagrams, 4, (1983), 349-351.
CrossRef
- Merica P. D., Waltenberg R. G., and Scot H., Bull. Am. Inst. Min. Metall. Eng., 150, (1919), 913- 949,
- Clark J. B., Acta Met., 13, (1965), 1281.
CrossRef
- Clark J. B., Acta Met., 16, (1968), 141.
CrossRef
- Fox F. A., and Farenhost E., Metallk Z., 34, (1942), 285.
- Talbot, A. M. and Norton, J. T., Transactions AIME, 122, (1936), 301-314.
- Leontis T. E. and Fanrenhost E., Metallk Z., 34, (1942), 285.
- Duly D., Simon J. P., Brechlet. Y., Acta Metall., 43, (1995), 101-106.
- Talbot A. M., and Norton J. T., Transactions AIME, 122, (1936), 301-314.
- Lagowski B., Am. Foundaryman’s soci. 79,(1971), 115.
- Crawley A.F., Metall. Trans,5, (1974), 949.
CrossRef
- Cellotto S., Acta mater., 48, (2000), 1775-1787.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal