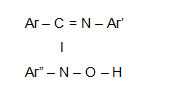IR Spectra, Magnetic and Thermal Studies of Copper (II) Complex of N-Hydroxy –N-(4-Chloro) Phenyl N’(4-Fluoro) Phenyl Benzamidine Hydrochloride
Sajila and Hemlata Mohabey*
Department of chemistry Government Digvijay Post Graduate College, Rajnandgaon (C.G.), India
DOI : http://dx.doi.org/10.13005/msri/110108
Article Publishing History
Article Received on : 04 Jul 2014
Article Accepted on : 23 Aug 2014
Article Published : 25 Aug 2014
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Copper (II) reacts with HCPFPBH and forms buff coloured precipitate insoluble in many organic solvents like absolute alcohol, ether, benzene, chloroform etc. The Solid complex has molecular formula (C19H13N2OFCl)2Cu which melts at 2020C with decomposition. The infrared spectra of the complex was recorded in the range 4000-450cm-1. The ligand molecule consists of a weak band at 2550 cm-1. This confirms the presence of azomethine nitrogen in the ligand. This band is absent in the IR Spectrum of the complex confirming the involvement of this group in complexation . The strong band of the ligand at 1640 cm-1 is due to C = NH+ group which shifts to lower frequency by 60 cm-1. This confirms the formation of C = N -----Cu bond. The N – O stretching mode shifts to higher frequency from 930 cm-1 to 960 cm-1 this confirms the formation of N – O – Cu bond by replacement of N – O – H proton by Copper.Cu – N band appears at 460 cm-1 which supports the formation of Cu – N bond. The positive value of volume susceptibility confirms that the complex is paramagnetic. TGA studies suggest that complex is thermally stable upto 2020C and melts with decomposition. At this temperature water molecules are absent which is also suggested by IR spectra of the complex. These studies support the use of N-Hydroxy-N-(4-Chloro) phenylN’-(4-Fluoro) phenyl benzamidine hydrochloride for gravimetric determination of Copper (II) in ores and alloys.
KEYWORDS:
Hydroxyamidine Copper Complex IR Spectra TGA
Copy the following to cite this article:
Sajila, Mohabey. H. IR Spectra, Magnetic and Thermal Studies of Copper (II) Complex of N-Hydroxy –N-(4-Chloro) Phenyl N’(4-Fluoro) Phenyl Benzamidine Hydrochloride. Mat.Sci.Res.India;11(1)
|
Copy the following to cite this URL:
Sajila, Mohabey. H. IR Spectra, Magnetic and Thermal Studies of Copper (II) Complex of N-Hydroxy –N-(4-Chloro) Phenyl N’(4-Fluoro) Phenyl Benzamidine Hydrochloride. Mat.Sci.Res.India;11(1). Available from: http://www.materialsciencejournal.org/?p=468
|
Introduction
Hydroxyamidines are organic reagents used for detection and determination of various transition metal ions. 1-5The reagent has azomethine nitrogen and –N-O-H group which makes it suitable chelating agent
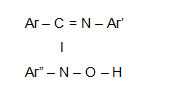
The properties of the reagent can be modified by suitable substitution in aromatic ring. Therefore a new hydroxyamidine has been synthesized by condensation of N-(4-Fluoro) phenyl benzamidoyl chloride in ether solution, like other hydroxyamidines this reagent also gives buff precipitate with Copper (II) in the pH range 2.5 to 10.5 which is easily filterable. The IR spectra, magnetic and thermal studies have been carried out.
Experimental
Apparatus
A single pan (dona) balance was used for weighing purpose, pH of the solution was measured with systronic pH meter type 321. IR Spectra was recorded in KBr on Perkin – Elmer – 1800 (FTIR) in the region 4000-450 cm-1. All the chemical used were of A.R. grade.
Method
Copper complex is precipitated instantaneously when reagent solution is added to cupric ions in the pH range 2.5 to 4.5. The complex is heavy, buff coloured and readily filterable. It is insoluble in water and alcohol, acetone, carbon tetra chloride and many other organic solvents. The excess of reagent is washed out easily. The complex was dried at 900– 1000C.
The elemental analysis data support 1:2 complex.
Element Calculated % Found %
C 61.40 59.29
H 3.50 3.88
N 7.54 7.32
Cu 8.56 8.42
The IR Spectra was recorded in KBr on Perkin-Elmer – 1800 FTIR in the region 4000-450 cm-1.
The ligand molecule consists of a weak band at 2550 cm-1. This confirms the presence of azomethine nitrogen in the reagent.6This band is absent in the IR Spectrum of Copper complex. Thus this group is involved in the complex formation.
A strong band at 1640 cm-1 due to C = N+H in the free ligand is shifted to 1580 cm-1 in the IR spectra of the complex.
This shifting of the band to lower frequency region is due to decrease in electron density in the azomethine linkage. This confirms the formation of – C = N —–Cu bond .
In the Copper complex N – O stretching band appears at 960 cm-1 which was 930 cm-1 in free ligand. The shift to higher frequency confirms formation of – N – O – Cu bond.
Metal – Nitrogen stretching band appears at 460 cm-1. According to Nakamota this Metal – Nitrogen band appears around 400 – 500 cm-1. Volume susceptibility8 measurements were done the positive value confirms paramagnetic nature of the complex.
TGA studies reveal that the complex starts decomposition around 2020C. There is no weight loss upto 202-0C indicating that water molecules are absent in the Copper complex. At 2100C about 5% weight loss is observed. Accelerated weight loss is observed upto 5900C where CuO is formed.
Conclusion
IR Spectra studies confirms Cu – N bonding and Cu – O bonding. The complex formed by N- Hydroxy – N – (4-chloro) phenyl N’(4-Fluoro) phenyl benzamidine hydrochloride and Cu (II) ion. The buff coloured water insoluble complex is stable upto 2020C and melts with decomposition at this temperature. The complex is paramagnetic. Thermal stability is confirmed upto 2000C by Thermogravimetric analysis. A quick, novel method can be developed using N – Hydroxy – N – (4-Chloro) phenyl N’-(4-Fluoro) phenyl hydroxyamidine hydrochloride as gravimetric reagent for estimation of Copper (II).
Acknowledgement
Authors are thankful to the Principal Dr.R.N. Singh Government Post Graduate College Rajnandgaon (C.G.) and Dr. Alok Mishra Head of Chemistry Department for providing laboratory facilities for the present work.
References
- K. Satyanarayanaand R.K.Mishra, and chem., 46 1605 (1974)
- K.Satyanarayanaand R.K.Mishra, Indian J.chem., 13, 295 (1975)
- H.Mohabey, P.K.Sharma and R.K.Mishra, Proc. Indian Acad. Sci 89 (2), 95 (1980)
CrossRef
- H.Mohabey and R.K.Mishra, J. Indian Chem. Soc, 57, 142 (1980)
- H.Mohabey and R.K.Mishra, J. Indian chem. Soc, 57, 562 (1980)
- K.Nakanishi, “Infrared Absorption spectroscopy” P. 122, Holden – Day Inc. San Francisco and Nakodo Co. Ltd., Tokyo (1962)
- K.Nakamoto,P.J.McCarthy, “spectroscopy and structure of Metal Chelate compound” John Wiley, New York (1968)
- P.W. Solwood, “Magneto Chemistry”, 2nd Ed., p.311, Interscience, New York (1965)
- W.W. Wendlendt, “Thermal Methods of Analysis” John Wiley and Sons, NY (1974)

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal