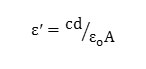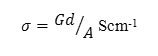Microstructures and Electrical Properties of HPMC/PVP Polymer Blend Films Complex with Ferric Chloride (FeCl3)
H. T. Ananda1, Thejas G. Urs2, Y. Prakash3, K. Hemalatha4, H. Somashekarappa4* and R. Somashekar2
1Department of Physics, Government College (Autonomous), Mandya – 571401, India.
2Department of Studies in Physics, University of Mysore, Manasagangotri, Mysore – 570006 , India.
3Department of Physics, V.V Puram Science College, Banglore - 560004, India.
4Department of Physics, Yuvaraja’s college, University of Mysore, Mysore – 570005, India.
DOI : http://dx.doi.org/10.13005/msri/110208
Article Publishing History
Article Received on : 18 Nov 2014
Article Accepted on : 26 Nov 2014
Article Published : 01 Dec 2014
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Microstructural studies on FeCl3 doped Hydroxypropyl methyl cellulose (HPMC)/Poly vinyl pyrrolidone (PVP) blend films were carried out using X-Ray diffraction studies. The XRD data revealed that the crystalline regions of the HPMC/PVP blend film decreases up to a certain percentage of FeCl3 and then increases. Electrical conductivity studies on these doped films suggest complex formation due to doping which affects microstructure and also ac conductivity of polymer films. All these results were analyzed and explained on the basis of micro structural modification of HPMC/PVP blends as function of dopant concentration.
KEYWORDS:
XRD; AC Conductivity; Polymer Composites
Copy the following to cite this article:
Ananda H. T, Urs T. G, Prakash Y, Hemalatha K, Somashekarappa H, Somashekar R. Microstructures and Electrical Properties of HPMC/PVP Polymer Blend Films Complex with Ferric Chloride (FeCl3). Mat.Sci.Res.India;11(2)
|
Copy the following to cite this URL:
Ananda H. T, Urs T. G, Prakash Y, Hemalatha K, Somashekarappa H, Somashekar R. Microstructures and Electrical Properties of HPMC/PVP Polymer Blend Films Complex with Ferric Chloride (FeCl3). Mat.Sci.Res.India;11(2). Available from: http://www.materialsciencejournal.org/?p=848
|
Introduction
Nowadays polymer blending is an easy and fastest way of getting a new material suitable for a specific purpose. Another way of tailoring the polymeric properties of an individual polymer or blends is by doping. The dopant can induce modifications in the molecular network and microstructures of the polymers. When a transition metal salts like FeCl3 and CoCl2 are doped, brings about a significant change in the physical properties including micro structural parameters, optical, electrical, thermal, and mechanical properties. These changes in physical properties, depends on the chemical nature of the dopant and the way in which they interact with the host polymer[1-3] Various researchers used different techniques to understand the micro structural properties and electrical properties of doped polymer films. Some of the studies are: effect of Iodine doping on PVA [4], temperature dependence of CuCl2 doped PVA[5], FeCl3 and CrCl3 doping effect on poly hydroxyl amine acid [6], effect of N H4I doping on PVA[7]. Proton conducting polymers are of great importance as they are used in solid state electro chemical power sources, sensors, electro chromic displays and fuel cells.[8, 9] The advantage of polymer electrolytes are their good mechanical properties, easy fabrication in to thin films of desirable size, and the ability to form good electrode/ electrolyte contact [10, 11] The main challenge in this field is to fabricate a low cost films with good mechanical and good electrical properties.
As we mentioned earlier number of literatures are available on PVA, PEO, polymer electrolytes complexed with various salts. But we have not noticed any article regarding HPMC or HPMC/PVP blend based polymer electrolyte. Hence we are reporting here the potential use of HPMC/PVP blend films as a host for in-organic salts in the preparation of polymer electrolyte .Hydroxypropyl methyl cellulose (HPMC), a bio- polymer derivative of cellulose available in powder form, water soluble and forms films. PVP a water soluble, synthetic polymer having good film forming nature. Both these were Microstructure parameters like crystallinity can be influenced by adding in-organic salts.
Materials and Methods
HPMC and PVP powdered samples were purchased from Loba chemicals Mumbai. Pure blend and doped films were prepared by using solvent cast method [12, 16] HPMC (5% wt) and PVP (2%wt) dissolved in 100 ml of distilled water separately. After complete dissolution the solutions were filtered using filter paper to remove undissolved particles.50ml of HPMC and 50ml of PVP were mixed and stirred continuously for 20 min with moderate speed. During stirring pre dissolved FeCl3 salt of 0.2 g was added to the blend solution and stirred continuously using magnetic stirrer for complete dissolution. After complete dissolution the solution was allowed for a while and then it is poured on to the clean glass plate and allowed to dry for a week. After complete dry, the film were peeled out of the glass plate and stored in desiccators to avoid moisture, following the same procedure other films of different concentration and salt were prepared. The prepared films appears to be yellowish transparent and they retains this transparency up to a certain percentage of the salt concentration in the blend matrix, after which they tend to be more opaque. This is an indication that the salt may possibly fully miscible creating a new polymer matrix constituted by one phase [17].
Experimental Procedure
X-ray Diffraction Measurements
The changes in the microstructure of the complexed films was examined by using Rigaku mini flex II diffractometer with Ni filtered Cukα radiation of wavelength 1.5406 Å, with graphite monochromator. The radiation was assessed at a voltage of 30 KV and 15mA. The dried samples were scanned in a 2θ range 6o to 40o.
AC Conductivity Measurements
AC conductivity measurements were made using LCR Hi Tester Hioki (Japan) 3532-50 programmable computer interfaced digital LCR impedance meter in the frequency range of 50 Hz to 5 MHz in room temperature.
Mechanical Properties
Test for mechanical properties of these polymer composite films were carried out using Lloyd’s Universal testing machine LRX Plug Model 5 KN. The obtained mechanical property parameters for these polymers are given in table 2.
Results and Discussions
XRD Studies
The pure HPMC/PVP blends and films of different FeCl3 concentrations were prepared. The prepared samples were investigated using WAXS method. From the diffraction pattern obtained it is found that a film consists of both crystalline and amorphous regions. In blends one sharp peak at 12o and one broad peak at 20o are observed. In salt doped films sharp peak disappears and only one broad peak at 2θ= 20o was observed. The intensity of the peak decreases and width increases. This is due to the disruption of crystalline regions of the blends by the FeCl3. Crystallinity was calculated from the ratio of the area under the crystalline peaks and the respective total area under the peaks, using PEAKFIT 4.1 software. Crystallinity decreases from 52.29(%) (Pure) to 21.30(%) (0.6g) and then increases to 26.52(%) for 0.8g salt doped film. Degree of crystallinity affects the electrical conductivity of the samples. The amorphous nature produces greater ionic diffusivity which increases the ionic conductivity. The greater ionic conductivity can be obtained in amorphous polymers that have a fully flexible backbone [18]. Micro structural parameters obtained are tabulated in tables 1.
It is observed that lattice strain in all the samples is around 0.1%-0.3%,the statistical percentage of deviation of parameters arises due to computation is around 0.06-0.38. With these parameters as input we have further refined these parameters against whole pattern (2θ=6o to 40o).The detailed theory of line profile analysis and whole powder pattern fitting method was discussed in our earlier papers [19-23]. The goodness of the fit between simulated and experimental profiles for the samples given in figure 1.It is worth noting that none of the other parameters such as lattice strain, stacking fault probability varied much during refinement, against the whole pattern data of the samples. The deformation fault probability values given in the table 1, indicates that the values are low, because there are too many layers between two successive deformation fault layers. These observed facts confirm the complexation of salt with blend matrix. Background corrections and instrumental broadening corrections were carried out before doing these above said analysis.
Table 1: Microstructural Parameters of HPMC/PVP Blend Films Doped With Fecl3.
|
Sample
|
2θ in deg
|
d in Å
|
<N>
|
D=<N>.d in Å
|
g (%)
|
αd
|
β
|
δ
|
α
|
|
HPMC/PVP
|
12.74
20.43
|
6.93
4.34
|
25.20
3.08
|
174.63
13.36
|
0.1
0.1
|
3.90E-05
1.70E-05
|
6.64E-06
4.46E-05
|
0.30
0.06
|
2.8
3.5
|
|
HPMC/PVP
+0.2% FeCl3
|
13.13
|
6.73
|
31.90
|
214.68
|
0.2
|
4.4E-05
|
9.89E-09
|
0.13
|
2.20
|
|
HPMC/PVP
+0.4% FeCl3
|
20.27
|
4.37
|
1.75
|
7.61
|
0.3
|
7.85E-07
|
1.91E-06
|
0.38
|
4.64
|
|
HPMC/PVP
+0.6% FeCl3
|
21.02
|
4.22
|
2.26
|
9.50
|
0.2
|
1.47E-08
|
8.15E-08
|
0.05
|
5.06
|
|
HPMC/PVP
+0.8% FeCl3
|
21.07
|
4.21
|
3.01
|
12.70
|
0.3
|
8.42E-06
|
7.78E-05
|
0.06
|
7.49
|
AC Conductivity
The average thickness and area of the prepared samples were found and, a piece of the sample was cut from the whole sample and silver paste was applied on both sides for good electrical contact between electrode-electrolyte. These samples were kept between the jaws of the sample holder for measurement of electrical conductivity and dielectric properties. Various parameters have been measured using Hioki (Japan) Model 3532-50 programmable computer interfaced digital LCR meter in the frequency range from 100 Hz to 5 MHz, at room temperature. The dielectric constant of a material can be calculated from the formula,
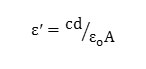
where c is capacitance, d is thickness, εo is the permittivity of free space, and A is the area of cross section of the sample and AC conductivity can be calculated from the equation below by which we have derived the conductivity for our samples.
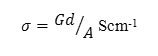
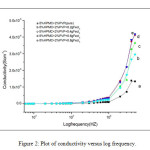
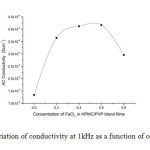
Where G is the conductance, d is thickness, and A is the area of cross section of the sample. Frequency dependence of ac conductivity was given in Fig 2. It is observed that ac conductivity increases with frequency. The variation of ac conductivity with concentration of FeCl3 is shown in the Fig 3. It is observed that the addition of FeCl3 significantly improves the ionic conductivity. The conductivity of pure HPMC/PVP blend is (1.34E-06), highest conductivity is found to be (4.17 E-06) for (0.6 g) sample. Above 0.6 g of FeCl3 the conductivity decreases due to re-crystallization of the sample. The increase of conductivity is due to the increase of mobile charge carriers due to increase of salt concentration and also to the amorphous nature of the polymer electrolyte.
Mechanical Properties
We have studied the changes in the mechanical strength of these polymer composites and the results obtained are shown in table 2. From the result it is seen that the tensile strength of the polymer composites decreases first with the concentration but it increased at the higher concentration. This indicates that the addition of FeCl3 to the polymer, affects its mechanical property due to the breaking of bonds between the polymeric molecules. But later at the higher concentration, the FeCl3 molecules form a network with the polymer and regain its strength.
Table 2: Mechanical Properties of HPMC/PVP And HPMC/PVP: Fecl3 Polymer Composites
|
Sample
|
Tensile Strength (MPa)
|
Stiffness (kN/m)
|
Young’s Modulus (MPa)
|
% Elongation at Fracture
|
|
HPMC/PVP
|
42.21
|
–
|
1480.31
|
3.70
|
|
HPMC/PVP + 0.2% FeCl3
|
12.74
|
945.85
|
1529.14
|
0.52
|
|
HPMC/PVP + 0.4% FeCl3
|
10.71
|
23.90
|
286.88
|
23.54
|
|
HPMC/PVP + 0.6% FeCl3
|
5.53
|
9.87
|
85.87
|
32.16
|
|
HPMC/PVP + 0.8% FeCl3
|
40.27
|
79.60
|
1415.65
|
14.28
|
Conclusions
X-ray diffraction study confirms that FeCl3 disturbs the crystalline nature of HPMC/PVP blend films. The increase in amorphous nature enhances the conductivity of the films. The highest conductivity found to be (4.17E-06 S/cm) for 0.6g FeCl3 doped HPMC/PVP blend film, which has least crystallinity of 21.30 (%) (from XRD). AC conductivity studies confirm that the conductivity increases with salt concentration. These films with more flexibility, good mechanical strength and degradable nature are good polymer electrolyte for electro chemical devices. Even though the conductivity of these polymer composites are not so profound as compared to the normal conducting polymers [24], it is seen that there is an increase in the conductivity of upto three times than that of the pure PVA due to doping.
References
- H. M. Zidan, (2003). Structural properties of CrF3, and MnCl2-filled poly(vinyl alcohol) films. Journal of Applied Polymer Science, vol. 88, no. 5, pp. 1115–1120.
CrossRef
- R.F.Bhajantri, V. Ravindrachary, A. Harisha, V. Crasta, S.P. Nayak, B. Poojary, (2006). Microstructural Studies on BaCl2 doped poly(vinyl alcohol), Polymer 47, 3591-3598.
CrossRef
- P.K.Khare, S.K. Paliwal, R. Kuraria, H.L. Vishwakarma, A. Verma, S.K. Jain, (1998). Electrical Conduction Mechanism in Solution Grown Doped Polyvinyl Pyrrolidone Films, Bull. Mater. Sci. 21, 139-147.
CrossRef
- B.Lobo., M.R.Ranganath, T.S.G.Ravi Chandran, G.Venugopal Rao, V.Ravindrachary, S.Gopal. (1999). Iodine-doped polyvinylalcohol using positron annihilation spectroscopy, Phys.Rev. B 59,13693-8.
CrossRef
- F.M.Hamdy.,Mohamed, Yasuo Ito, Ali M.A, El-Sayad, EsamE,Abdel-Hady, (1996) Positron Annihilation in Polyvinylalcohol Doped with CuCI2. Polymer 37,1529.
- A.Mazzrona, N.Mostafa, E.Gomaa, M.Mohsen, the Use of Positron Annihilation Lifetime Technique to Study the Effect of Doping Metal Salts on Polyhydroxamic Acid Polymers, J.Appl.Polym.Sci, 81, 2095 (2001).
CrossRef
- M.Hema, S.Selvasekerapandian, G.Hirankumar, A.Sakunthala, D.Arunkumar, H.Nithya. (2009), Structural and Thermal Studies of PVA:NH4I, Journal of Physics and Chemistry of solids,70,1098-1103.
CrossRef
- M.Mika, M.Paidar, B.Klapste, M.Masinova, K.Bouzek, Vondrak, (2007) Hybrid inorganic–Organic Proton Conducting Membranes for Fuel Cells and Gas Sensors. J.Phy.Chem.Sol. 68 775.
CrossRef
- B.Scrosati, (1993) Applications of Electroactive Polymers,Chapman and Hall, London.
CrossRef
- Rajendran S, Mahendran O, Mahalingam T, (2002) Thermal and Ionic Conductivity Studies ofPlasticized PMMA/PVdF Blend Polymer Electrolytes. Eur. Polym. J. 38. 49 – 55.
CrossRef
- Nader Binesh, S.V.Bhat, (1998) VTF to Arrhenius Crossover in Temperature Dependence of Conductivity in (PEG) xNH4ClO4 Polymer Electrolyte. J.Poly.Sci. (B) 36 1201-1209.
CrossRef
- Tanabe T Okitsu N, Tachibana A and Yamauchi K. (2002) Preparation and Characterization of Keratin-Chitosan Composite Film. Biomaterials, 23, 817-825.
CrossRef
- Konno.H, Honda T, Alonzo D, Yaylor L. (2008) Effect of Polymer Type on the Dissolution Profile of Amorphous Solid Dispersions Containing Felodipine. Eur J Pharm Biopharm, 70(2):493-499.
CrossRef
- Kim S J, Park S J, Kim I Y, Lee Y H, Kim S I. (2002) Themal characteristic of poly(vinylealcohal) and poly (vinylpyrrolidone), INPs, J App.Polymsci, 86, 1844-1847.
- Biswas.A.,Willet JL,Gordon SH, Finkenstadt VL, Cheng HN, (2006) Complexation and Blending of Starch, poly(Acrylicacid) and poly(N-vinylpyrrolidone). Carbohydr polym. 65, 397-403.
CrossRef
- Ping Z, Nguyen QT, Neel.J. (1994) Investigation of Poly(Vinyl Alcohol)/Poly(N-vinyl-2-pyrrolidone) Blends, 3. Permeation Properties of Polymer Blend Membranes. Macromol.Chemphys. 195, 2107-2116.
CrossRef
- Nyamweya N and Hoag S W, (2000) Assesment of Polymer-Polymer interaction in Blends of hpmc and Film Forming Polymers by Modulated Temperatue Differential Scanning Calorimetry. Pharm.Res.17, 625-631.
CrossRef
- Mohammad A.A, Mohamed N.S, Yahya M.Z.A, Othman R, Ramesh S, Alias Y, A.K.Arof. (2003) Ionic Conductivity Studies of Poly(Vinyl alcohol) alkaline solid polymer electrolyte and its use in nickel–zinc cells. Solid state Ionics, 156, 171-177.
CrossRef
- Hall I.H and Somashekar R (1991) The determination of crystal size and disorder from the X-ray Diffraction Photograph of Polymer Fibres. 2. Modelling intensity profiles. J.Appl.Cryst., 24, 1051-1059.
CrossRef
- Somashekar R, Hall I.H and Carr P.D (1989) The Determination of Crystal size and disorder from X-ray diffraction photographs of polymer fibres. 1. The accuracy of determination of Fourier coefficients of the intensity profile of a reflection. J.Appl.Cryst. 22, 363(1989).
CrossRef
- F.G. Souza Jr, B.G. Soares, G.L. Mantovani, A. Manjunath, H. Somashekarappa, R. Somashekar, Siddaramaiah (2006) Blends of styrene butadiene styrene TRI block copolymer/polyaniline-Characterization by WAXS, Polymer, 47, 2163–2171.
CrossRef
- P.Parameshwara, T.Demappa,M.Mahadevaiah, Y.Prakash, H.Somashekarappa, Byrappa K and R.Somashekar. (2012) Polymeric degradation of water soluble chitosan/HPMC films using WAXS data. Material research Innovations, 16, 126-129.
CrossRef
- S.Divakara, S.Madhu,and R.Somashekar, (2009) Stacking faults and microstructural parameters in non-mulberry silk fibres, Pramana, 73(5) 927.
CrossRef
- Thejas Urs G, Mahadevaiah D and Somashekar R (2014) Studies on Structural and Conducting Properties of Goethite Nanoparticles Doped HPMC Polymer Films. Journal of Polymers, 201464: doi:10.1155/2014/201464.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal