I. Bsoul*
Physics Department, Al al-Bayt University, Mafraq 130040, Jordan.
Corresponding author:
E-mail: Ibrahimbsoul@yahoo.com
DOI : http://dx.doi.org/10.13005/msri/120105
Article Publishing History
Article Received on : 21 May 2015
Article Accepted on : 11 Jun 2015
Article Published : 11 Jun 2015
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this work, a single phase strontium ferrite sample was mechanically milled for 60 h. It has been found that the crystallite size reduced by 85%. A dramatic drop (from 4500 to 1600 Oe) in the coercive filed was also observed. The observed reduction in the coercivity was attributed to the reduction of the single domain particle size.
KEYWORDS:
Mechanical milling; Strontium ferrite; Coercive field
Copy the following to cite this article:
Bsoul I. Effect of Mechanical Milling on the Magnetic Properties of SrFe12O19. Mat.Sci.Res.India;12(1)
|
Introduction
Strontium hexaferrite (SrFe12O19) is a hard magnetic material that possesses important magnetic properties such as high saturation magnetization and high coercive field. A significant change in the coercive field of hexaferrite usually can be achieved by the substitution of Fe3+ ions by either cations or cations combination such as Ga, Al, Cr, Co-Ti, Ti-Ru [1-6]. This work is concerned with mechanical milling effect on the coercivity of strontium hexaferrite powder compound.
A single phase powder sample of SrFe12O19 was prepared by solid state method. For further details of fabrication please refer to our previous publication [1-3]. SrFe12O19 powder was mechanically milled in a planetary ball-mill (Fritsch Pulverisette-7) at 250 rpm for 60 h with ball to powder ratio of 16:1. XRD, SEM and magnetic measurements were carried out to investigate the initial un-milled sample (IS) and milled for 60 h sample (MS). Also Rietveld refinement was carried out to refine XDR data using FULLPROF suite package.
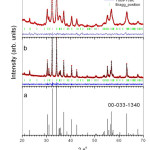
Figure 1 shows XRD patterns for the IS, MS and standard pattern ICDD file (00-033-1340) for hexagonal strontium ferrite SrFe12O19 [7]. One might observe that no secondary phase is present in either IS or MS. The MS sample is still a single phase even after 60 h of high-energy mechanical milling. Rietveld refinement for IS and Ms is also shown at Figure 1. Bragg factor (RB) and the goodness of fit quality (x2) are shown in Table 1. The values of x2 for the samples are less than 1.3, which indicates a good fit. The structural parameters for the samples are also tabulated in Table 1. A negligible change in lattice parameters (less than 0.18%) occurs as a result of mechanical milling. The average crystallite size for the samples was calculated using.
Figure1: Standard ICDD pattern for SrFe12O19 (a), XRD patterns for un-milled sample (b) and milled for 60 h sample (c).
Table 1: Bragg factor, χ2, lattice parameters a and c, unit cell volume and average crystallite size of SrFe12O19.
|
x
|
RB
|
χ2
|
a = b (Å)
|
c (Å)
|
V (Å)3
|
D (nm)
|
|
IS
|
2.69
|
1.103
|
5.8814
|
23.0355
|
690.0658
|
83
|
|
MS
|
3.63
|
1.21
|
5.8771
|
23.0257
|
688.7679
|
12
|
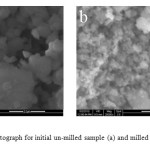
Scherrer formula [8] and the results are shown in Table 1. The average crystallite size for IS is 83 nm, while for MS was 12 nm. So the crystallite size records a 85% reduction as a result of mechanical milling for 60 h. Figure 2 shows a SEM photograph of initial un-milled and milled for 60 h samples. The particle size for IS seems to be around the single domain size of 460 nm
Figure2: SEM photograph for initial un-milled sample (a) and milled for 60 h sample (b).
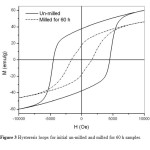
Figure3: Hysteresis loops for initial un-milled and milled for 60 h samples.
which reported by Rezlescu et al. [9]. While the particle size for MS seems to be smaller than that for IS by an order of magnitude. Figure 3 shows hysteresis loops for initial un-milled and milled for 60 h samples. The coercivity decreased significantly from 4500 Oe for the un-milled sample down to 1600 Oe for the sample milled for 60 h. In view of the fact that the particle size determined from SEM imaging for the un-milled sample is generally of the order or smaller than the critical single domain size of 460 nm [9], we conclude that the particles in this sample are generally single domain particles. Upon milling for 60 h, the particle size dropped significantly by almost an order of magnitude. The coercivity of a single domain particle was reported to follow a typical behavior with particle size, such that the coercivity has a maximum value at the critical single domain size, and decreases with decreasing particle size down to zero at the superparamgnetic critical size [10]. Consequently, the observed reduction in the coercivity is attributed to the reduction of the single domain particle size in the milled sample.
References
- Bsoul I., Mahmood S. H., Abdel-Fatah Lehlooh, J. Alloys Compd., 2010, 498, 157.
CrossRef
- Bsoul I., H. Mahmood S., J. Alloys Compd., 2010, 489, 110.
CrossRef
- Bsoul I., Mahmood S. H., Abdel-Fatah Lehlooh, Ahmed Al-Jamel, J. Alloys Compd,. 2013, 551, 490.
CrossRef
- Bsoul I., Jordan Journal of Physics, 2009, 2 (2), 95.
- Bsoul I., Mahmood S. H., Jordan Journal of Physics, 2009, 2 (3), 171.
- Awawdeh M., Bsoul I., H. Mahmood S., J. Alloys Compd., 2014, 585, 465.
CrossRef
- International Centre for Diffraction Data (ICDD), File 00- 033-1340.
- Warren B. E., X-Ray Diffraction, Addison-Wesley, Reading, Massachusetts, 1969.
- Rezlescu L., Reslescu E., Popa P.D., J. Magn. Magn. Mater., 1999, 193, 288.
CrossRef
- Charles S., Popplewell J., Ferromagnetic liquids, Handbook of Ferromagnetic Materials 2, 1980, 509.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal