J. Chidanandappa1, K. Eswara Prasad2, K. Balaraju3 and V. N. Mani3
1National Remote Sensing Centre, Balanagar, Hyderabad-500037, India
2Department of Mechanical Engineering, Jawharlal Nehuru Technological University, Hyderabad-500072, India
3Centre for Materials for Electronics Technology, Hyderabad 500051, India
DOI : http://dx.doi.org/10.13005/msri/120106
Article Publishing History
Article Received on : 26 Apr 2015
Article Accepted on : 12 May 2015
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this study, directional freezing technique was employed to prepare pure gallium antimonide (GaSb) compound and purified samples were also been characterized to ascertain their and purity level and crystalline quality. The select impurities that were targeted for reduction include Al, Ca, Mg, Sb, Si, Sn, Ge, Cu, Fe, Zn and Ag. Controlled melting and freezing conditions were followed to homogenize the sample. A multi-pass directional freezing experiment on the pre-homogenized GaSb sample was conducted. The experimental conditions and parameters such as temperature gradient, sample tube movement rate, crucible geometry was established and optimized. The prepared homogenous GaSb sample was cleaned under contamination controlled conditions aided by 1000 class clean room and 100 class clean benches. Then the homogenous sample was loaded in the high quality quartz ampoule for further purification process. The sample containing ampoule was subjected to melting-freezing scheme with 20 passes cycle and the 4N+ pure GaSb crystalline ingot thus prepared. The purified and crystallised sample was characterized for its purity employing ICP-MS and crystalline quality through XRD techniques respectively and the results are discussed.
KEYWORDS:
Gallium antimonide,Purification,Directional freezing,Purity analysis
Copy the following to cite this article:
Chidanandappa J, Prasad K. E, Balaraju K, Mani V. N. Preparation of Gallium Antimonide and its Characterization. Mat.Sci.Res.India 2015;12(1)
|
Copy the following to cite this URL:
Chidanandappa J, Prasad K. E, Balaraju K, Mani V. N. Preparation of Gallium Antimonide and its Characterization. Mat.Sci.Res.India 2015;12(1). Available from: http://www.materialsciencejournal.org/?p=1746
|
Introduction
GaSb is a potential III-V semiconductor material required for transistors, laser diodes, photo-detectors and fiber optic communication and other related opto-electronic applications [1]. GaSb has the band gap of 0.17 eV at 300K, that corresponds to IR wave length (6.2 um) and thus find in variety of IR related optoelectronic applications. Gallium antimonide is an important substrate material due to its favorable properties such as low electron mass, high mobility, lattice match with various ternary and quaternary III-V compounds. GaSb bulk crystals are grown by Czochralski and Bridgman methods. The Bridgman technique poses various inherent problems, in regard to control of poly-crystalline and structural defects etc. In this paper, we report some of the results pertaining to the synthesis, preparation and characterization of pure gallium antimonide (GaSb). Gallium and antimony were used as input starting materials to prepare pure GaSb compound. Optoelectronics device applications demand gallium, indium and arsenic, antimony purity in the level of 99.99999 (7N), which is referred to as IC grade. For 7N pure metal, the total amount of the impurities in gallium, indium, arsenic, antimony must be less than 100 ppb. The metallic impurities of most concern in IC grade gallium, antimony are Al, Fe, Cu, Si, Zn, Ge, and Cd, In, Sn etc. These elements along with carbon, oxygen and other gaseous impurities present should be less than 1 to 10 ppb in gallium, indium and arsenic, antimony. The impurities present in the starting material contribute shallow and deep energy levels in the energy gap (GaAs, InSb, and GaSb), which eventually leads to common device failure, when GaSb, InSb wafers used for fabrication of GaSb, InSb device. Therefore, the impurities, which are in the ppb/ppt levels in metals, alloys and compound should be analyzed with great accuracy and consistency through employing advanced analytical techniques such as Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Glow Discharge Mass Spectrometry (GDMS), Residual Resistivity Ratio Analysis etc.
Directional freezing and crystallization
Refining and crystal growth techniques are mainly used for purification, fractional crystallization of materials and growth of crystals. The material to be purified and crystallized must be in a solid state. In directional solidification or fractional crystallization, a narrow slice of the material is melted to form a molten zone which is made to pass through the entire length of material. Since the concentration of impurities in the solid crystallized from molten zone is different from the remaining in the liquid. The directional solidification or crystallization processes leads to the preparation of material with a purity of greater than 99.9999% with reasonable pure 4N starting material. Physico- and thermo-chemical and fractional crystallization considerations for efficient refining through directional freezing. The following factors are affecting and influencing the refining process: a) zone size and length of sample, b) zone travel rate, diameter of the sample tube, b) temperature gradient, c) thermal conductivities of phases, d) latent heat of fusion, e) density differences between solid and liquid, f) speed of crystal formation, g) surface tension of liquid, h) tendency of liquid to attain ambient temperature [2]. The purification obtained by directional freezing can be defined in terms of segregation coefficient ‘K’ which is the ratio of concentration of impurities in the crystallizing solid phase to the concentration of impurity in the liquid phase. In practice, however some of the impurity rich liquid is stopped in the solidifying substance and this gives greater value of ‘K’ than that obtained from the theory. Some impurities may be seen trapped amongst the vapor bubbles in the sample. However, with most materials the value of ‘K’ is less than one and purity of material is considerably increased after a few refining cycles. In some cases ‘K’ is greater than one end impurity is concentrated at the beginning of the sample instead of at the end.
Directional freezing system and growth ampoule design
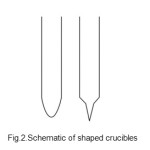
The indigenously developed directional freezing system has two heating zones and provides a temperature up to 10000 C. The furnace temperature is controlled to +5 0 C accuracy and is capable of handling of 250-400g sample. The directional freezing system includes a vertical gradient two zone furnace, translational and rotational assemblies. The furnace provides two temperature zones namely, a high temperature and graded-temperature zones. The crucible moves from the high temperature portion of the furnace to the graded temperature lower portion. Furnace temperature profile moves related to a downward translated and rotated crucible, leading to the controlled unidirectional solidification of the material. Impurities are driven and travel (bottom to top) parallel to the melt-solid interface resulting in the bottom portion of the ingot to get purified. Translation assembly consisting of gear motor/console, shafts and limit switch integrates and supports the crucible (sample) holder rod; so that the crucible is lowered into the vertical furnace with desired low translational rate. The rotational assembly supports the crucible holder and rotates the crucible at 15-50 rpm during both homogenization and the directional solidification of the material. It is well proven that, the efficient crystallization process depends on the crucible type, shape and design. The most obvious desirable characteristics of a crucible are that, its presence should not in turn contaminate the material that is taken for further refinement. Also, it is highly desirable that the crucible should have smaller coefficient of thermal expansion than the material. It is should also have a smaller thermal conductivity than the material intended for crystallisation. In this work, special quartz glass ampoules of 1 mm thick having different diameters and shapes were got fabricated and used. The ampoules were also cleaned and dried well in order to prevent the presence of contamination.
Fig.1. Directional freezing system
Fig.2.Schematic of shaped crucibles
Experimental
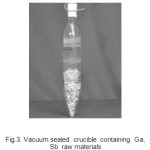
Homogenization, synthesis and directional freezing experiments on 4N+/ 5N pure GaSb (four batches-250 grams level) were carried out with a view to study the segregation characteristics of select Al, As, Bi, Ca, Mg, Sb, Si, Sn, Ge, Pb, Cu, Fe, Zn and Ag impurities in the GaSb major matrix. For homogenization, treated Ga and Sb metals were loaded in to high quality quartz ampoule and sealed under vacuum of 5 x 10-6 Torr level. The sample was then subjected to melting (7500 C)-solidification(100 C)-re-melting (7500 C) with 12 hrs pass (es) and 3 cycle(s) scheme. Homogenized samples were then taken out from the ampoule in a clean room (1000 class)/100 class bench environment and crushed, grounded and finely powdered samples were prepared. The powdered samples were then further loaded in high quality quartz ampoule and sealed under vacuum of 10-6 Torr level. The sample was directionally solidified employing the directional melting- freezing- solidification processes (710-720 0C) with 24 hrs pass time, travel rate of 25-35 cm.hr-1, rotation rate of 35-45 rpm and 3 cycles- pass (es) scheme.The purified sample was then cut into three portions-top, middle and region segments and samples taken from different regions were analyzed employing ICP-MS technique and through XRD technique for crystalline quality.
Fig.3. Vacuum sealed crucible containing Ga, Sb raw materials
Fig.4 . Purified and crystallised GaSb
Class clean material processing /handling: Gallium antimonide is relatively less toxic material. Clean environment (10000/1000 class) and clean laminar flow benches (100 classe) were used in the pre and final purification processes and preparation of samples for analysis of GaSb to achieve the required purity. Required safety precautions were taken and glove box facilities were used while handling high pure materials, chemicals. The crucibles and starting material-5N purity Ga, Sb sample(s) were first treated employing the cleaning procedures such as, a) treating the crucibles with electronic grade trichloro ethylene, b) washing, rinsing, pickling with electronic grade acetone, methanol and nano-pure water, c) preparation of ultra pure/electronic grade aqua regia (1:1 ratio HCl+HNO3), d) filling up and treating of beakers/crucibles with electronic grade aqua regia (HNO3 + HCl) – for 2hrs, e) cleaning of the crucible with aqua regia solution, f) treating with electronic grade supra pure HCl- oxide layer removal, g) drying the metal.
Results and discussion
The use of proper shape of the ampoule is crucial in the case of directional freezing and crystallisation technique to enable have single nucleus which would then propagate to form structured crystal . The formation of single nucleus is more certain. The ampoules with council shapes readily enhance this chance. The conical tip in the ampoule is the point of initiation of the nucleation, solidification and controls further crystallisation. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) technique was employed to analyse and estimate the specific targeted impurities level( Li, Na, Cr, Mn, Fe, Ni, Sr, Cd, Sn, Hg and Pb) . Purity analysis results of refined of GaSb sample are presented in table 1 and Purity analysis results of refined of GaSb sample are presented in table 2.
Table 1. Purity test results staring materials Ga and Sb – ICP-OES( in ppm)
|
Element
|
Ga
|
Sb
|
|
Li
|
1.522
|
2.882
|
|
Na
|
2.200
|
4.700
|
|
Cr
|
5.190
|
6.210
|
|
Fe
|
1.925
|
1.723
|
|
Ni
|
9.540
|
9.512
|
|
Sr
|
4.650
|
14.622
|
|
Cd
|
7.640
|
5.971
|
|
Sn
|
8.510
|
9.678
|
|
Hg
|
6.320
|
8.543
|
Table2. Purity test results of purified GaSb
|
Element
|
Ga
|
|
Li
|
0.282
|
|
Na
|
0.435
|
|
Cr
|
0.180
|
|
Fe
|
1.865
|
|
Ni
|
0.387
|
|
Sr
|
0.333
|
|
Cd
|
6.943
|
|
Sn
|
0.020
|
|
Hg
|
0.245
|
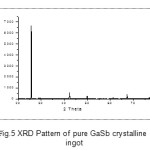
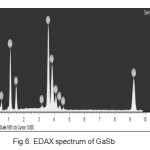
The qualitative trend of impurity (ies) segregation on to the different locations of the rod (ends) was observed. It was also observed that, the effect of experimental conditions and parameters such as number of passes, zone length, temperature gradient, environmental cleanliness and over all contamination control plays a crucial role in achieving consistent results. The crystalline quality of prepared GaSb sample was also studied by X-ray Diffractometry (XRD) and the results are shown in fig 5. The typical EDAX spectrum is also shown in figure 6. The results and our part findings were also in tune and agreement with the reported works [3-5].
Fig.5 XRD Pattern of pure GaSb crystalline ingot
Fig.6. EDAX spectrum of GaSb
Conclusion
3N+ pure gallium and antimony were used to synthsise GaSb and the prepared GaSb was purified. Refined GaSb samples were characterized by ICP-MS and it is confirmed that purity of the GaSb is 4N+ pure with respect to select targeted impurities. Crystalline perfection of the purified GaSb was also studied by EDAX and XRD and found that material is in crystalline form.
References
- Van Welzenis R G and Ridely B K 1984 Solid State Electronics 27 113
CrossRef
- V N Mani, K. Ghosh and K. Balaraju, Mat.Sci.Res. India 5(1), 89-94 , (2008)
CrossRef
- N K Uday Shankar and H L Bhat, Bull Mater.Sci vol 24, No.5 October 2001, pp 445-453
CrossRef
- Dutta, P.S , Rao K. S. R. K, Bhat H. L , and Vikram Kumar, 1995,Applied Physics A 61 149
CrossRef
- P. S. Dutta, K. S. Sangunni, H. L. Bhat, and Vikram Kumar, J. 1996 , Ind. Inst. Sci. 76, 235.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal