M. A. Gadyal*1, K. S. Venkatesh2
1Department of Materials Science Gulbarga University, Gulbarga.
2Research Guide,Department of Materials Science Gulbarga University, Gulbarga.
DOI : http://dx.doi.org/10.13005/msri/120114
Article Publishing History
Article Received on : 14 Mar 2015
Article Accepted on : 08 Apr 2015
Article Published : 08 Apr 2015
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
The polyaniline belongs to the group of electronically conducting polymers. The graphite is anisotropic, being a good electrical and thermal conductor within the layers. One of the more important groups of materials in our lives today is composite material. The nano-composites provide reinforcing efficiency because of their high aspect ratios In this paper, synthesis and characterization polyaniline- graphite as a novel eco-friendly nano-composite material is reported.
KEYWORDS:
Polyaniline; graphite; nano-material; composites
Copy the following to cite this article:
Gadyal M. A, Venkatesh K. S. Synthesis of Polyaniline-Graphite Nano-Composites. Mat.Sci.Res.India;12(1)
|
Introduction
Materials science studies the fundamental physical and chemical basis for the controlled combination of atoms to form new compounds, phases, and micro structures, as well as the characterization of the resulting structures and their properties. One of the more important groups of materials in our lives today is composite material. The man made composite materials, that are a three-dimensional combination of at least two chemically distinct materials, with a distinct interface separating the components, created to obtain properties that cannot be achieved by any of the components acting alone1.
Composite materials have many advantages over monolithic materials, because their mechanical, chemical and electrical properties can be tailored as per the requirements for particular applications. Sometimes the properties of a composite can be strikingly different from the properties of the constituent materials. For example, two materials with positive thermal expansion coefficients can be combined to create a porous structure to give a composite with a negative thermal expansion coefficient2.
Usually, composite materials have the components, called the matrix and the filler. The matrix is the component that holds the filler together to form the bulk of the material. It usually consists of various epoxy type polymers but other materials may also be used. The filler is the material that has been impregnated in the matrix to lend its advantage (physical and chemical change) to the composite. The four main manmade composite groups are: metal matrix composites polymer matrix composites, ceramic matrix composites and conducting composites. The conducting composites are the mixture of conducting and insulating phases or the mixture of high and low conducting materials2.
The electrical properties of these conducting composite materials are of great technological and scientific interest. The electrical conductivity of such materials can be altered to a few orders of magnitude by increasing the conducting phase above the percolation threshold. Such dramatic changes in the electrical properties can be utilized for various applications.
The discovery of electrically conducting polymers, inventers A. Heeger, A. MacDiarmid and H. Shirakawa awarded with the Nobel Prize in chemistry in the year 2000. The three winners established that polymer plastics could be made highly conducting by suitable doping3. Certain conjugated organic polymers such as polyaniline, polypyrrole poly-p-phenylene, polythiophene and polyacetylene show very high value of conductivity4.
The Polyaniline (PANI) belongs to the group of electronically conducting polymers, which are of great interest for practical applications at present. Conductive PANI has been studied extensively because of its ease of synthesis. It can be synthesize by chemically or electrochemically. The numerous methods employed to synthesize this material have produced several products, which differ in their nature and properties and must represent the result of multitude of polymerization mechanisms of aniline. The aniline polymers are on limelight because of their environmental stability, controllable electrical conductivity and interesting redox properties associated with the nitrogen atoms in the main chain. The PANI has numerous applications5,6.
Graphite (GRA) is a naturally occurring, steel-gray to black, crystalline form. The graphite consists of carbon layers and each carbon layer is called graphene layer. The graphite is anisotropic, being a good electrical and thermal conductor within the layers and a poor electrical and thermal conductor perpendicular to the layers. The carbon atoms in graphite are strongly bonded together in layers. Because the bonds between the layers are weak, other atoms can easily fit between them 7,8.
Since many important chemical and physical interactions are governed by surfaces and surface properties, a nanostructured material can have substantially different properties from a larger-dimensional material of the same composition. In the case of particles and fibers, the surface area per unit volume is inversely proportional to the material’s diameter, thus, the smaller the diameter, the greater the surface area per unit volume9,10.11.In the present work, nano-composites containing two different weight % of graphite powder filler material were synthesized and XRD and SEM analysis were done.
Materials and Methods
Natural flake graphite with an average diameter of 500 µm washed with acetone and dried for three days. The solution of 0.2 M aniline (C 6H 5NH 2) and 0.1 M ammonium persulphate [(NH4) 2– S2O8] was prepared in 1 M HCl in two separate flasks using triple distilled water and the measured quantity of graphite powder is added[7-10]. These solutions were slowly mixed under continuous stirring at room temperature, the initial colorless solution slowly turned green. After completion of the reaction, the resultant product was repeatedly washed in Buchner funnel with distilled water till filtered solution turned from acidic to neutral. Finally, it was washed with acetone to remove low-molecular weight intermediates of aniline (brown colored solution). The dried material was grinded to fine powder and stored in a sealed bottle for further processing.
The two samples of weight percentage of ratio PANI:GRA=90.55:9.45 and 80.762:19.238 are prepared from above mentioned procedure.
Results
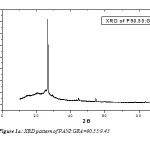
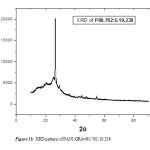
The X-ray diffraction (XRD) patterns were obtained using CuKa radiation at a scanning rate of 2.0mm/min., using a voltage of 45 kV and a current of 40 mA. Fig. 1a and 1b shows the X-ray diffraction patterns of as-prepared PANI:GRA=90.55:9.45 and 80.762:19.238 composites respectively. In Fig.1a one diffraction peak is located at θ= 13.2603º and in Fig.1 b the diffraction peak is located at θ=13.2712º.
The figures 2a and 2b are the respective SEM images of the PANI: GRA=90.55:9.45 and 80.762:19.238 composites.
Discussion
From the XRD pattern particle sizes were determined using FWHM method. The sizes were 20.8985 nm and 18.447 nm of PANI:GRA 90.55:9.45 and 80.762:19.238 composites respectively. The SEM analysis that the samples appear like two dimensional sheets. From the histogram the mean diameter of first sample was 22.28 nm with standard deviation 1.9 nm and for second sample was 21.22 nm with standard deviation 1.7 nm. Polyaniline-graphite composites of different weight percentage were prepared in powder form and analysis reveals the particle are nano in size. Hence we succeed in synthesis of nano-composites of polyaniline and graphite.
Acknowledgement
This work was supported by research center, Department of materials science, Gulbarga University Gulbarga (Karnataka –India).
References
- J.Stejskal,IUPAC,Pure and Applied Chemistry.74, 857-867 (2002).
CrossRef
- Graeme.W.Milton “The Theory of Composites”45(2003).
- John O’ M Bakris and Amulya K.N. Reddy “Modern electrochemistry II edition”63(2003)
- J. Njugunaand K. Pielichowski Journal of Materials Science 39 4081 – 4094(2004)
- S.C Ragevendra, Syed Khasim, M. Revenasiddappa, N.Ambikaprasad and A.B. Kulkarni et al. Material Science, 26, No.7, pp, 733 739,( 2003).
- J. Njugunaand K. Pielichowski Journal of Materials Science 39 4081-4094(2004)
- . D.C.Trivedi “Polyanilines”C.P.453( 2004).
- D.D.L.CHUNG, Journal of Materials Science, 37 1475(2002).
CrossRef
- Meng Chen, Chunyu Du, Long Wang, Geping Yin, Peng Fei Shi, Int.J. Elecrochmic. Sc. &819-829(2012)
- Farzana Hussain, Mehdi Hojjati, Masami Okamoto and Russell E. Gorga Journal of Composite Materials; 151140;( 2006)
- Luo, J.J. and Daniel, I.M.. Characterization and Modeling of Mechanical Behavior of Polymer/Clay Nanocomposites, Compos. Sci. Technol., 63(11): 1607–1616(2003).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal