Protective Effect on Metal Substrate of Epoxy Resins Containing Corrosion Inhibitor Embedded Into Suitable Nanocarriers
Introduction
Corrosion process consists in the deterioration of metal materials through chemical interaction with the external environment. One of the most common strategies used to protect metallic structures against corrosion consists on the application of protective organic coatings. The polymer layers create a barrier and, thus, avoid the contact of water and ion species with the metallic substrate offering a passive protection. However the use of coatings suffers from the negative effects of external agents such as UV radiation, temperature and mechanical action such as the formation of scratches or cracks. These lead to the opening of pores and crack propagation in which water and salts can diffuse and reach the metal interface, ending up in corrosion initiation. In order to prolong the life time of metallic structures, the additional use of an active protection is highly desirable. To this aim, corrosion inhibitors can be embedded into the polymeric protective layer.1,2 Among them, 2-mercaptobenzothiazole (MBT) is one of the most widely used corrosion inhibitors for many metals3 in applications fields such as aeronautic, automotive and shipbuilding industries. However, it is well known that MBT can be photodegraded by solar irradiation and it can be critical for the corrosion protection.4 Furthermore the corrosion inhibitors are prone to leach from the polymer layer thus leading to rapid exhaustion of their anti-corrosion activity. To overcome these drawbacks, a valid approach is represented by the designing of active coatings wherein the inhibitors are encapsulated in micro/nano carriers.5 In this coatings the nanoparticles act as a reservoir and allow the slow release over time of the inhibitor.
In the present paper the effect of epoxy resins loaded with 2-mercaptobenzothiazole embedded into two different types of nanocarriers on the corrosion protection of commercial steel substrates has been investigated. The two nanocarriers are halloysite nanotubes (HNTs) and Zn-Al-layered double hydroxides (LDH). Halloysite is a naturally mineral clay normally described as a gibbsite octahedral sheet (Al(OH)3), which is modified by siloxane groups at the outer surface, and has a 1:1 Al:Si ratio and stoichiometry Al2Si2O5(OH)4·nH2O.6 The size of halloysite tubes varies from 500-1000 nm in length and 15-100 nm in inner diameter.6 Halloysite exhibits a range of morphologies, and its structure depends on crystallization conditions and geological occurrences.7 Halloysite nanotubes are attractive materials due to their availability, relatively low cost and wide range of applications. It has been used as support for immobilization of catalysts such as metal-complexes8,11 and for the controlled release of anti-corrosion agents, herbicides and fungicides.12,17 Herein, with the aim to control the release of the anticorrosion agent, we propose to modify the anticorrosion filled HNT particles with a chemicalcross-linker, such as copper ions, able to produce a network acting as a physical ending stopper.
Layered double hydroxides (LDHs general formula of M2+1-x–M3+x(OH)2(An–)n/x·mH2O), also sometimes termed anionic or hydrotalcite clays are a class of layered materials consisting of positively charged brucite-like layers and the charge compensating interlayer exchangeable anions.18 Synthesis and chemical modification of LDHs offer attractive research opportunities, mostly due to their good environmental stability and simple intercalation/deintercalation chemistry. Tremendous potential applications of LDHs in catalysis, medicinal materials, waste removal, polymer nanocomposite, and drug delivery have already been reported.18,19
The ability of LDH to act as a generic anion delivery system leads tothe possibility to immobilize organic anion inhibitors by intercalation between hydroxide layers. Herein two LDHs, characterized by different morphology, i.e. LDH rose and flat particles have been used to embed the MBT corrosion inhibitor. They differ from the dimension of particles, in particular the LDHs with the rose morphology, range from 600 to 1500nm, while the LDHs with the flat morphology, range from 8000 to 15000nm. Furthermore, the first appear as aqueous wet powders, whereas LDH flat are dry powders.
The MBT-loaded HNTs and LDH particles were characterized using Fourier transform infrared (FTIR) spectroscopy and thermogravimetric analysis. The MBT release kinetics from the loaded nanoparticles were investigated using UV-vis spectroscopy and the obtained data were correlated with the protective effect of MBT-containing resins on metallic substrates which has been studied by visual observation during accelerated corrosion tests.
Materials and Method
Halloysite nanotubes were provided from Imerys Ceramics (UK).Zinc-aluminum 2-mercaptobenzothiazole hexahydrates rose wet powders ([Zn0.67Al0.33(OH)2](MBT)0.33 · 2,4 H2O) and Zinc-aluminum 2-mercaptobenzothiazole hexahydrates flat dry powders ([Zn0.67Al0.33(OH)2](MBT)0.3(CO3)0.015 · 2,9 H2O), were purchased from Prolabin & Tefarms.r.l. (Perugia, Italy) already loaded with corrosion inhibitor molecules. A commercial ferritic steel was used as substrate material for the coating. The 2-mercaptobenzothiazole, used as the corrosion inhibitor and sodium chloride, used for corrosion studies were purchased from Sigma-Aldrich (Italy). The polymeric coating was obtained by using a liquid epoxy resin (DGEBA, provided from ELANTAS Camattini S.p.A.) cured with Jeffamine D230 (Sigma Aldrich, Italy), a poly propylene glycol bis-2-aminoprolyl ether used as hardener. All the chemicals were used as received.
Experimental section
Procedure for loading of MBT in HNT nanotubes
HNT powders were sieved using a 63µm mesh and then dried at 120°C overnight to remove adsorbed water.Afterwards, the HNTs were firstly treated under vacuum for 24h to remove the air entrapped in the lumens and then mixed with the saturated MBT solution in acetone in order to load the corrosion inhibitor. After 30min, the vial was taken from the vacuum jar and the suspension was brought in an ultrasonic bath, at room temperature for few minutes to avoid the aggregation of HNT and improve their dispersion in the saturated solution. The vial containing nanotubes and MBT solution was placed in a jar and kept under vacuum for 30min. Then the suspension was recovered and kept under stirring for 30min. This process was repeated three times to increase the loading efficiency. The MBT-loaded HNTs were separated by centrifugation (4000 rpm for 3 min), rinsed rapidly with water to remove the free MBT and dried via oven at 60°C overnight.
It has been reported that to avoid burst release and to prolong the release time of organic molecules from HNT lumen, it is possible to form a complex between the active molecule loaded in HNTs and copper ions (Cu2+),8-11, 14,10 which contribute to form a network that acting as physical stopper. The MBT cross-linking was realized by treating the loaded nanotubes with a copper ions solution which, diffusing into the tube openings, formed an insoluble metal-MBT complex capping the tube ends.20 For this purpose, MBT-HNT particles were exposed for 1 min to the bulk aqueous solution containing copper ions (CuSO4) under continuous stirring. The obtained nanotubes, coded MBT-Cu-HNT, were separated from the solution by centrifugation and dried in oven at 60°C overnight.
Preparation of the active epoxy coating
The production of epoxy-based coatings has been obtained by using the Thinky instrument, to remove the air entrapped in the mixtures, following the three cycles reported in Table 1. Jeffamine D230 was added to the homogeneous systems in stoichiometric amount with respect to the epoxy groups present in the resin, and the same Thinky cycles were applied two times. Active epoxy-based coatings were prepared by adding suitable amounts of MBT-loaded nanocarriers in order to obtain in all the films an MBT amount equal to 1%w/w with respect to epoxy weight.
The mixtures were deposited on the surface of the commercial steel substrates through Elcometer Film Applicator, using a thickness of 30 microns. Finally, epoxy-based coatings were cured in the oven at 170°C for 15 minutes. A pristine epoxy coating was also prepared as control sample.
The following samples were prepared: pristine epoxy coating (labelled as DGEBA+D230); coating filled with MBT_HNT (labelled as DGEBA+D230+MBT_HNT); coating filled with MBT_Cu_HNT (labelled as DGEBA+D230+MBT_Cu_HNT); coating filled with MBT_LDHr (labelled as DGEBA+D230+MBT_LDHr) and finally coating filled with MBT_LDHf (labelled asDGEBA+D230+MBT_LDHf).
Preparation of metal substrate surfaces
A commercial ferritic steel sheet was cut into rectangular specimens (8.5 x 5.5 x 0.2 cm3), abraded with silicon carbide (SiC) emery sheet up to 1000 grit, cleaned with sodium hydroxide solution (0.1M), deionized water, and finally in ethanol to remove oil and dust. Then, the substrates were coated using the different epoxy-based coatings.
Characterization
The morphology and structure of the inorganic carriers were investigated using Scanning electron microscopy (SEM), a Leica model S440 (Leica Microsystems GMbH, Germany) operating at 20 kV. The powders were placed on the support and coated with gold with a sputter coater (Emscope SC500, UK) before they were observed under the microscope.
Active nanoparticles were characterized by infrared spectroscopy analysis and thermogravimetric analysis.FT-IR spectra were collected with a Nicolet FT-IR spectrophotometer using a KBr pellet over a range of 4000– 400cm-1 with a resolution of 2cm-1. The thermogravimetric analysis (TGA) was performed by using a TGA 500 (TA Instruments), submitting the samples to a heating run from 30°C up to 900°C at a heating rate of 10°C/min, under nitrogen flux.
The release kinetics were determined by mixing the MBT-loaded nanocarriers powder in corrosive solution ([NaCl] = 0.05M) at pH=7 at room temperature and sampling the solution at prefixed time intervals. The amount of released 2-mercaptobenzothiazole as a function of time was monitored by UV−Vis analysis by using a Agilent Technologies Cary 60 UV−Vis spectrophotometer at absorption intensity peak at ca. 322nm. A calibration curve of MBT was obtained by plotting the absorbance vs. MBT concentration between 1 and 10ppm.
Table 1: Thinky cycles.
|
Speed
|
2000rpm
|
600rpm
|
2000rpm
|
|
Vacuum
|
yes
|
no
|
yes
|
|
Time
|
2min
|
4min
|
4min
|
Accelerated anticorrosion studies were performed by scratching the polymer coating with a cross cut, thus exposing the coated surface of steel substrate to corrosive conditions. The scratched coated substrate was dipped into corrosive solution ([NaCl] = 0.05M)for 1minute every day, and kept in a closed chamber containing a saturated solution of NaCl. Trough visual observation it has been possible to evaluate the time needed to visualize the occurrence of corrosion spots and their extension and amount.
Results and Discussion
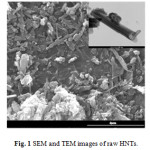
Figure 1 shows SEM/TEM images for pristine nanocarriers. HNTs particles possessed a typical hollow tubular structure and both ends were open. The internal and external diameters of the HNTs ranged from 15 to 30nm and 30–100nm, respectively.The SEM micrograph reported in the Figure 2(left side) shows LDH submicron particles aggregated to form the rose morphology sized between 600-1500 nm. Whereas in Figure 2(right side) is showed the hydrotalcite particles with a flat morphology, with a hexagonal shape and size between 8-15 µm.
Figure 1: SEM and TEM images of raw HNTs.
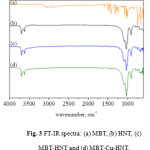
Figure 3 displays the FTIR spectra of HNTs samples before and after loading procedure with MBT. Compared to the unloaded sample, MBT-HNT and MBT-Cu-HNT samples exhibit some new FTIR peaks, such as several peaks around 1496cm-1attributed to the benzene ring stretching, the C=N stretching at 1322cm-1, typical of the MBT spectra, and the C-C-C bending absorption band at 1011cm-1.
Figure 2: SEM of two hydrotalcite particles, on the left shown LDH rose and on the right the flat type.
Figure 3: FT-IR spectra: (a) MBT, (b) HNT, (c) MBT-HNT and (d) MBT-Cu-HNT.
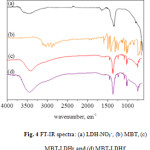
The infrared spectrum of the LDH systems were shown in Figure 4. The LDH characteristic infrared absorption bands were observed at around 3500cm-1, likely ascribed to the brucite-like layer (OH stretching vibration), at 1623cm-1 due to the hydroxyl stretching and at 1384cm-1 due to NO3–.21 In the spectra of the loaded nanocarriers, it is possible to identify the MBT bands at 3000cm–1 due to the C-H deformation, the C=N stretching at 1322cm-1, and the C-C-C bending absorption band at 1011cm-1.
Figure 4: FT-IR spectra: (a) LDH-NO3–, (b) MBT, (c) MBT-LDHr and (d) MBT-LDHf.
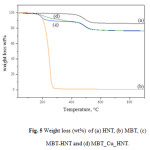
MBT loading efficiency was determined by thermogravimetric analysis. In Figure 5the TGA diagrams of HNT, pristine MBT and the two MBT-loaded Halloysite nanocarriers have been reported.The MBT curve shows only one weight loss which is completed at about 275°C.HNT curve exhibits one degradation step at about 500°C which is attributed to the dehydroxylation of the matrix [22]. By comparing the weight loss of the loaded halloysite with that of an unloaded sample,it is possible to calculate the loading efficiency by taking into account the difference of residue at 800°C. The obtained data show a load efficiency equal to about 10% w/w for both nanocarriers. The results also confirm that in the case of HNT the loading efficiency does not change after Cu2+ solution treatment.
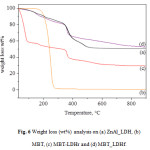
TGA curves of pristine MBT and LDH systems are shown in Figure 6. As mentioned above, the MBT curve shows only one weight loss which is completed at about 275°C. In the case of MBT-LDHr particles (wet state), three thermal degradation steps can be observed: the first step is related to water evaporation; the second one between 100 and 180°C is due to the loss of interlayer hydrogen bonded water molecules;the third step corresponds to the overlapping of layer de-hydroxylation and to the decomposition of intercalated anion.
Figure 5: Weight loss (wt%) of (a) HNT, (b) MBT, (c) MBT-HNT and (d) MBT_Cu_HNT.
Figure 6: Weight loss (wt%) analysis on (a) ZnAl_LDH, (b) MBT, (c) MBT-LDHr and (d) MBT_LDHf.
The amount of MBT released from the active nanoparticles in corrosive NaCl solution (0.05M, pH=7)at 25°C was monitored as a function of time.In Figure 7a and 7b, release profiles of MBT from pristine and complexed halloysite nanotubes,and from rose and flat LDHs were respectively reported.
Figure 7: Release kinetics of MBT from neat and complexed HNTs (a) and from rose and flat LDH (b). (c) MBT UV spectra from different nanocarriers.
From Figure 7a it can be inferred that the release of MBT from the active HNT nanoparticles takes place very quickly within 5 hours and can be considered complete in 24 hours. Also in the case of HNTs loaded with MBT and treated with copper solution, the complete release of MBT is very fast and is completed after 24 h. However, the amount of inhibitor released at the equilibrium (34%w/w) is less than that initially loaded into the particles. This can be likely ascribed to a very stable complex formed between MBT and Cu ions,14 which prevents the total release in the surrounding aqueous medium.
As for LDH nanocarriers, it is worth noting that the mechanism of MBT release is related to the ion-exchange capacities in the chloride-based aggressive environment. From Figure 7b, it can be noticed that after 15 days MBT release from both rose and flat LDHs has not reached the asymptotic value and that MBT anions are released to an higher extent from the LDH flat nanocarrier with respect to the amount released from LDH rose system. Since LDH flat consists in a dry powder where as the LDH rose are wet particles, this different releasing behavior can be ascribed to the presence of electrostatic interactions between MBT ions and water molecules, which increase the MBT stability in the LDH rose carrier, thus decreasing the released amount.
Figure 7c shows the UV-vis spectra of pristine MBT and MBT compound released respectively from HNT and LDH systems. It’s observed that the spectrum of MBT from HNTs is comparable to that of pristine one. In contrast the spectrum of MBT released from LDHs systems is somewhat different. This difference may be tentatively ascribed to the presence of anionic MBT compounds which are embedded in the LDHs structures.
Anticorrosive effect of MBT during accelerated corrosion process of metallic substrates was studied by visual observation of corrosion spots in the scratched area of the steel substrate coated with epoxy resin. In Figure 8 the optical photographs of the scratched steel substrates coated with neat and active epoxy resins, at time zero and after 30 days of contact with corrosion solution, are reported.
Pictures show that pristine epoxy-based coating, not containing the corrosion inhibitor, is not able to protect the metallic substrates from the corrosion process which rapidly and extensively occurs (Fig. 8a), after one day aging. In the case of DGEBA+D230+MBT_HNT resin (Fig. 8b), the presence of MBT absolutely hinders the corrosion process up to 30 days of exposure to corrosive solution. Whereas, when MBT is embedded into HNTs treated with copper solutions, the corresponding active coating is not effective in protecting the scratched coated substrate from corrosion (Fig. 8c). This behavior is supported by the release data which show that MBT forms a stable complex with Cu ions and thus it is not available to be released (only 40%w/w of loaded MBT is released). It’s likely that the released MBT amount is not enough to establish a protective coating in the scratched zone. On the other hand, interesting results are reported when the metal substrate is coated with resins containing MBT-loaded LDHs. Figure 8d) and 8e) show no visible degree of corrosion up to 30 days of exposure. In this case, even if the release of the active compound is not complete, the released anionic MBT (as confirmed by UV spectra) is very effective to prevent corrosion process even at low concentrations. As previously reported, LDH are anion-exchange compounds and the anticorrosive MBT anions can be exchanged by the corrosion-active ions such as chlorides present in the aggressive environment. In this case, the high performance in corrosion protection is mainly due to the twofold action of the LDH nanostructured epoxy-based coating which steadly released the effective active MBT anions compound over exposure time and also provides a good barrier to the penetration of corrosive and aggressive species.
Figure 8: Pictures of the scratched steel substrates coated with the various epoxy resins after 0 days (left) and 30 days (right) of contact with the corrosive NaCl solution. (a) DGEBA+D230, (b) DGEBA+D230+MBT_HNT, (c) DGEBA+D230+MBT_Cu_HNT (d) DGEBA+D230+MBT_LDHr and (e) DGEBA+D230+MBT_LDHf.
Conclusions
The preliminary results reported in this paper confirm that the designing and preparation of active coating is a valuable approach to contrast the mechanism of corrosion of metal substrate and mayensure a long-term stability of the metallic objects. It has been shown that the coatings based on LDH particles loaded with anionic anticorrosion inhibitors MBT are more effective in protecting the metallic substrate with respect to the coating based on HNT particles filled with MBT. The different behavior is ascribed to both the different releasing mechanisms and the different released species. In fact in the case of HNT particles, the inhibitor is quickly released as neutral MBT specie in the aqueous solution and, thus, it shows a stabilization effect of the metal substrate.On the other hand, the results obtained for the coatings containing MBT loaded into copper-treated HNT particles confirm that when the MBT is released at lower concentration is not able to promote the formation of a stable anticorrosion layer. In the case of LDHs, the anionic form of the inhibitor is slowly released from the particles, because that depends on the ion exchange process,thus providing steadily new anticorrosive molecules which result more effectual in contrasting the process corrosion.
Acknowledgments
This work was supported by the Italian Ministerodell’Istruzione, dell’Università e dellaRicerca (MIUR), and Ministerodello Sviluppo Economico within the framework of PON02_00029_3205863‘PRADE’. The authors would like to thank Prolabin Perugia (Italy) for their support in the LDH synthesis preparation.
References
- Zheludkevich M. L., Poznyak S. K., Rodrigues L. M., Raps D.,Hack T., Dick L. F., Nunes T., Ferreira M. G. S. Corrosion Science. 2010;52:602-611.
CrossRef
- Zheludkevich M. L., Tedim J., Ferreira M. G. S. ElectrochimicaActa. 2012;82:314-323.
CrossRef
- Kartsonakis I. A., Dragatogiannis D. A.,Koumoulos E. P., Karantonis A.,Charitidis C. A. Materials and Design. 2016;102:56-67.
CrossRef
- Serdechnova M., Ivanov V. L., Domingues M. R. M., Evtuguin D. V., Ferreira M. G. S and Zheludkevicha M. L. Phys.Chem.Chem.Phys. 2014;16:25152-25160..
CrossRef
- Kartsonakis I. A., Balaskas A. C., Koumoulos E. P., Charitidis C. A., Kordas G. C. Corrosion Science. 2012;57:30-41.
CrossRef
- Guimarães L., Enyashin A. N., Seifert G.,Duarte H. A. Journal of Physical Chemistry C. 2010;114(26):11358–11363.
CrossRef
- Joussein E., Petit S., Churchman J., Theng B., Righi D., Delvaux B. Clay Minerals. 2005;40:383-426.
CrossRef
- Abdullayev E., Price R., Shchukin D., Lvov Y. Appl. Mater. Interf. 2009;1:1437–1443.
CrossRef
- Joshi A., Abdullayev E.,Vasiliev A., Volkova O., Lvov Y. Interfacial Nanoarchitectonics. 2013;29:7439-7448.
- Wei W., Minullina R., Abdullayev E., Fakhrullin R., Mills D.,Lvov Y. RCS Adv. 2014;4:488-494.
- Nakagaki S.,Wypych F. Journal of Colloid And Interface Science. 2007;315:142-157.
CrossRef
- Machado G. S.,Dias de Castro K. A. F., Wypych F., Nakagaki S. Journal of Molecular Catalysis A: Chemical. 2008;283:99–107.
CrossRef
- Shchukin D., Möhwald H. Small. 2007;3:926−943.
- Abdullayev E., Abbasov V., Tursunbayeva A.,Portnov V., Ibrahimov H., Mukhtarova G and Lvov Y. Appl. Mater. Interfaces. 2013;5:4464−4471.
CrossRef
- Moazeni N., Mohamad Z., IzzatiFaisal N. L., Tehrani M. A., Dehbari N. J. Appl. Polym. Sci. 2013;955-960.
CrossRef
- Fix D., Andreeva D. A.,Lvov Y. M., Shchukin D. G.,Mohwald H. Adv. Funct. Mater. 2009;19:1720-1727.
CrossRef
- Lvov Y. M., Shchukin D. G.,Mohwald H., Price R. R. ACS Nano. 2008;2:814-820.
CrossRef
- Newman S. P., Jones W. New Journal of Chemistry. 1998;22:105-115.
CrossRef
- Dong Y., Wang F., Zhou Q. Journal of Coatings Technology and Research. 2014;11:793-803.
CrossRef
- Abdullayev E., Lvov Y.Journal of Materials Chemistry. 2010;20:6681–6687.
CrossRef
- Kang H., Shu Y., Li Z., Guan B.,Peng S., Huang Y., Liu R. Carbohydrate Polymer. 2014;100:158-165.
CrossRef
- Horvath E., Frost R. L.,Mako E., Kristof J., Cseh T. Thermochimica Acta. 2003;404:227-235.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal