Tushar Shamba Anvekar,*  Vrajeshwari Rajendra Chari
Vrajeshwari Rajendra Chari  and Hari Kadam
and Hari Kadam 
Department of Chemistry, St. Xavier’s College, Mapusa, Goa – 403507, India
Corresponding Author Email: tsanvekar@yahoo.co.in
DOI : http://dx.doi.org/10.13005/msri/140211
Article Publishing History
Article Received on : 9 Sept 2017
Article Accepted on : 20 Sept 2017
Article Published : 16 Oct 2017
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Nano ZnO was synthesised by green approach employing aqueous extract of Adulsa and Lemongrass leaves. XRD suggested hexagonal wutzite structure for these prepared ZnO. Synthesised nanoparticles efficiently catalysed Biginelli reaction.
KEYWORDS:
Biginelli reaction; FTIR; XRD; Green Synthesis; ZnO nanoparticles
Copy the following to cite this article:
Anvekar T. S, Chari V. R, Kadam H. Green Synthesis of ZnO Nanoparticles, its Characterization and Application. Mat.Sci.Res.India;14(2)
|
Copy the following to cite this URL:
Anvekar T. S, Chari V. R, Kadam H. Green Synthesis of ZnO Nanoparticles, its Characterization and Application. Mat.Sci.Res.India;14(2). Available from: http://www.materialsciencejournal.org/?p=6100
|
Introduction
Nanoparticles are gaining importance in various fields such as health, biomedical science, chemicals industries, food & feed, cosmetics, environmental, drug and gene delivery, energy science, electronics mechanics and space industries.1-2
Design of processes that reduces or eliminates generation of hazardous substances is the key principle for sustainable chemistry. Developing green methods for synthesising nanoparticles is a major focus of present research scenario.3-7
Available literature forecasts that the nanoparticle synthesis using medicinal plants, micro-organisms and algae and other as source has been unexplored and underexploited.8-16
Materials and Methods
Preparation of Extract: A fresh leaf of Adulsa or Lemongrass leaves were collected and washed several times with deionized water, grinded and boiled for 30 min. till the colour of solution changes from watery to light yellow and filteres to get the extract. The filtrate is used as a stabilizing (capping) agent.
Experimental procedure: For the synthesis of naoparticles, Adhatoda/Lemongrass leaves extract (50 mL) was taken and heated to 80 °C with vigorous stirring. ZnNO3.6H2O (5 g) was added to the solution under heating. The paste thus obtained was heated in ceramic crucible at 400 °C for 3 h in air. Nano ZnO was obtained as white powder.17-18
Characterisation: XRD analysis was performed on Philips XPert Pro diffractometer. JEOL JSM-6360LV Scanning Electron Microscope was used to study surface morphology of nanoparticles. FTIR were recorded on ThermoFischer FTIR instrument with KBr.21-22
Catalytic Application
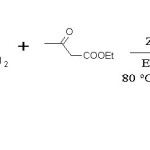
Urea (15 mmol, 0.90 g), Ethylacetoacetate (13 mmol, 1.69 g), Benzaldehyde (10 mmol, 1.06 g), prepared ZnO catalyst (0.2 g), Ethanol (10 mL) were refluxed for 3 hr. (Scheme 1) Mixture was then filtered in crushed ice and crude solid product as separated by filtration and washed with water. Product was purified by recrystallisation with Ethylacetate and structure was confirmed by comparing melting point and IR data.23-25
Scheme 1: Biginelli reaction
Results and Discussion
X-ray Diffraction (XRD)
The technique provides valuable information of structure, different phases and preferred crystal orientation. The pallets sintered at 400 C were finely powdered and used for XRD analysis. Inter-atomic spacing (d), lattice parameter (a), and particle size (D) were calculated for the highest intensity peak having miller indices as (hkl) for the above sample.
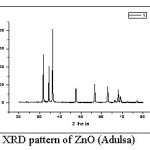
Figure 1: XRD pattern of ZnO (Adulsa)
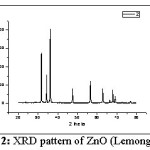
Figure 2: XRD pattern of ZnO (Lemongrass)
Fig. 1 shows XRD pattern for ZnO prepared using adulsa leaves extract. The peaks are indexed as 34.45º (100), 31.78º (002), 36.235º (101), 56.55º (102), 47.51º (110), 62.84º (103), 66.06° (200), 67.95º (112), 69.01° (201) and 78.82° (202). These peaks correlate to the hexagonal wurtzite structure of ZnO without any impurity peaks. The average crystallite / particle size as calculated by Debye-Scherrer equation was found to be 28 nm.
Fig. 2 shows XRD pattern for ZnO prepared using Lemongrass leaves extract. These peaks are indexed as 31.74° (100), 34.40° (002), 36.23° (101), 47.52° (102), 56.59° (110), 62.82° (103), 66.30° (200), 67.88° (112), 69.00° (201) and 76.93° (202). This pattern also correlates to the Hexagonal wurtzite structure of ZnO. The average crystallite / particle size was found to be 50 nm.
Scanning Electron Microscopy (SEM):
Fig. 3 and 4 shows SEM images depicting the morphologies of nano ZnO obtained by Adulsa and Lemongrass resp.
Figure 3: SEM Images of ZnO (Adulsa)
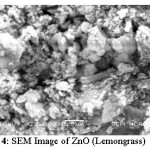
Figure 4: SEM Image of ZnO (Lemongrass)
Figure 3 shows the formation of irregular spherical ZnO nanoparticles, and change of the morphology of the nanoparticles. This SEM image indicates irregular morphology. Some particles are in the range of 85-95 nm.
Figure 4 shows the formation of spherical ZnO nanoparticles, and changes of the small granular size of zinc oxide nanoparticles are formed. Spherical morphology of the nanoparticles, mixed with some chunky particles due to agglomeration. It is seen from the image that the zinc oxide nanoparticles range from 85-98 nm and there are also some nanoparticles are formed in the range of 500 nm.
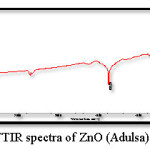
Figure 5: FTIR spectra of ZnO (Adulsa)
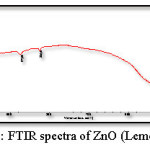
Figure 6: FTIR spectra of ZnO (Lemongrass)
Figure 5 and 6 shows the FTIR spectrum of the ZnO nanoparticles synthesized by green method, this spectrum shows accordance with the literature spectrum. IR graph depict that the adhatoda (adulsa) extract acts a stabilization (capping agent). Adhatoda sample is rich in alkaloids, tanins, saponins, phenolic, and flavonoids. The chemical constituents of lemongrass extract are terpene associated to aldehyde, alcohol and ketones. All these constituent promote these lemongrass extract as a stabilizing agent. These biomolecules helped in stabilization (capping) of the synthesized ZnO NPs.
Table 1 presents the performance of prepared ZnO catalyst for Biginelli reaction giving dihydropyrimidinone product. (M.P. 207 °C, Lit. 206-208 °C)23-25
Catalytic Activity
Table 1: Catalytic activity
|
Catalyst
|
Time (Min)
|
% Yield
|
|
ZnO (ADULSA)
|
180
|
64
|
|
ZnO(LEMONGRASS)
|
180
|
75
|
|
COMMERCIAL ZnO
|
180
|
66
|
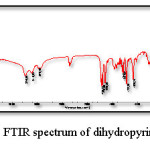
Fig. 7 displays the IR spectrum of dihydropyrimidinone product obtained from Biginelli reaction while Table 2 correlates its characteristic absorption peaks with probable vibrational modes.
Figure 7: FTIR spectrum of dihydropyrimidinone
Table 2: Characteristics bands
|
Observed Peak cm-1
|
Stretching mode
|
|
3245
|
N-H asymm
|
|
3115
|
N-H symm
|
|
2979
|
C-H
|
|
1725
|
C=O Ester
|
|
1701
|
C=O Amide
|
|
1645
|
C=C
|
|
1089
|
Monosubstituted Aromatic Ring
|
Conclusion
In this study we report a simple, biological and low cost approach for nano Zinc oxide using various leaves extract asadhatoda (adulsa), lemongrass as the reducing agent. They are employed as stabilizing agents. The prepared Zinc oxide nanoparticles were characterized by XRD, SEM and FTIR. XRD patterns of ZnO nanoparticles prepared with the green synthesis method using leaves extracts of adhatoda (adulsa), lemongrass, neem, meethi, tulsi, shows average particles size calculated as in the range of 25-50 nm by using Scherer formula. SEM image indicate irregular morphology with particles are in the range of 85-100 nm. Also, the synthesized ZnO catalyst was used in Biginelli reaction to prepare 3,4-Dihydropyrimidinones from ethylacetoacetate, benzaldehyde and urea with around 75 % product yield.
Acknowledgement
Authors thank National Institute of Oceanography, Goa for analysis.
References
- Meruvu S., Hugendubler L., Mueller E. Regulation of adipocyte differentiation by the zinc finger protein ZNF638. Journal of Biological Chemistry. 2011;286(30):26516-23.
CrossRef
- Manoj K., Sukumar D., Amit K., Sinha M. P. Determination of nutritive value and mineral elements of five leaf chaste tree and malbar nut (adhatoda vasica nees) Academic Journal of Plant Sciences. 2013;6(3):103-108.
- Manoj K., Amit K., Sukumar D., Sinha M. P. Photochemical screening and antioxidant potency of adhatoda vasica and vitex negunda. The Bioscan. 2013;8(2):727-730.
- Facts about Malbar nut (Asticia adhatoda), Encyclopedia of life, retrieved 03/01/2013.
- Linares S., Gonzalez N., Gomez E., Usubillaga A., Darghan E. Effect of the fertilization, plant density and time of cutting on yield and quality of the essential oil of Cymbopogon citratus Stapf; Rev. Fec. Agron, (LUZ). 2005;22:247-260.
- Padalia R. C.,Verma R. S. Comparative volatile oil composition of four ocimum species from north india, Nat. Prod. Res. 2011;25(6):569-75.
CrossRef
- Maribel G. G., Jean D., Stephan G. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity, International Journal of Chemical and Bimolecular Engineering. 2009;2(3): 104-111.
- Buazar F., Bavi M., Kroushawi F., Halvani M., Khaledi-Nasab A., Hossieni S. A. Potato extract as reducing agent and stabiliser in a facile green one-step synthesis of ZnO nanoparticles, Journal of Experimental Nanoscience. 2016;11(3):175-184.
CrossRef
- Janaki A. C., Sailatha E., Gunasekaran S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;144:17-22.
CrossRef
- Yang X., Geng D., Weng L. J., Han Y., Yang W. J. Green Synthesis of Silver Nanoparticles Using Chitosan for 4-Nitrophenol Reduction. Advanced Materials Research. 2012;479:332-335.
CrossRef
- Veluswamy P., Sathiyamoorthy S., Chowdary K. H., Muthusamy O., Krishnamoorthy K., Takeuchi T., Ikeda H. Morphology dependent thermal conductivity of ZnO nanostructures prepared via a green approach, Journal of Alloys and Compounds. 2017;695(25):888-894.
CrossRef
- Vanathia P., Rajiv P., Narendhran S., Rajeshwari S., Pattanathu, Rahman K.S.M., Venckatesh R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach, Materials Letters. 2014;134:13-15.
CrossRef
- Edhaya Naveena B., Prakash S. Biological Synthesis of Gold Nanoparticles Using Marine Algae Gracilaria Corticata and its Application as a Potent Antimicrobial and Antioxidant Agent, Asian J Pharm Clin Res. 2013;6(2):179-182.
- Liu R. P., Su W. M., Wang C. A. Preparation and Characterization of Al2O3-Based Composite Membranes by Sol-Gel Method, Key Engineering Materials. 2015;655:108-113.
CrossRef
- Sangareswari M., Meenakshi Sundaram M. High UV light performance for the degradation of Rhodamine B dye by synthesized Bi2S3ZnO nanocomposite, Appl. Phys. A. 2017;123:364.
CrossRef
- Srirangam G. M., Parameswara Rao K. Synthesis and Charcterization of Silver Nanoparticles from the Leaf Extract of Malachra Capitata (L.), Rasayan J. Chem. 2017;10(1):46-53.
- Jeong M. C., Jae K. S., Seung M. P. Characterization of zinc oxide nanoparticles grown by laser ablation of Zn target in near water.Bull. Korean Chem. Soc. 2009;30(7):1616-1618.
CrossRef
- Doungporn Y., Kanittha B. and Wiyong K. Preparation of zinc oxide nanostructure by solvothermalmethods,Journal of Microscopy Society of Thailand. 2009;23(1):75-78.
- Hongxia L., Jiyang W., Hong L., Huaijin Z., Xia L. Zinc oxide prepared by sol gel methods,Journal of Crystal Growth. 2005;275(1–2):943-946.
- Vinay Kumar B., Naik H. S. B., Girija D., Vijaya Kumar B. ZnO nanoparticle as catalyst for efficient green one-pot synthesis of coumarins through Knoevenagel condensation; Journal of Chemical Sciences. 2011;123(5):615–621.
CrossRef
- Chennupati J., Stephen P. Zinc Oxide Bulk, Thin Films and Nanostructures Processing, Properties and Application, Elsevier. 2006;ISBN: 978-0-08-044722-3.
- Philip D. Green synthesis of gold and silvernanoparticles using Hibiscus rosasinensis, Physica E. 2010;42:5:1417-24.
CrossRef
- Maryam B., Hassan S. Nanosized magnesium oxide as a highly effective heterogeneous base catalyst for the rapid synthesis of pyranopyrazoles via a tandem four-component reaction, Arabian Journal of Chemistry. 2011;4(2):159–162.
CrossRef
- Phucho I. T., Nongpiur A., Tumtin S., Nongrum R., Nongkhlaw R. L. Recent progress in the chemistry of dihydropyrimidinones, Rasayan J. Chem. 2009;2(3):662-676.
- Anvekar T. S., Korgaonkar K. K., Kadam H. Synthesis, characterization and catalytic application of MgO supported metal catalysts in synthesis of dihydropyrimidinone, Res. J. Material Sci. 2017;5(2):1-6.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Vrajeshwari Rajendra Chari
Vrajeshwari Rajendra Chari  and Hari Kadam
and Hari Kadam 
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal