M. Ravindar Reddy 1*  , M. Jaipal Reddy 2 and A. R. Subrahmanyam1
, M. Jaipal Reddy 2 and A. R. Subrahmanyam1
1Dept.of.S and H, MVSR Engineering College, Hyderabad, India, 501510
2Dept.of.Physics, Palamuru University, Mahaboobnagar, India, 509001
Corresponding author Email: ravimurvakonda@gmail.com
DOI : http://dx.doi.org/10.13005/msri/140206
Article Publishing History
Article Received on : 21 Oct 2017
Article Accepted on : 3 Nov 2017
Article Published : 16 Nov 2017
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
This paper is a report of a study conducted on Structural, thermal and optical Properties of pure PMMA, pure PEO and PMMA-PEO-LiClO4 polymer blend electrolyte thin films. These films were prepared using solution casting technique and characterized by X-ray Diffractometer (XRD), Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR) and Differential Scanning Calorimetry (DSC). XRD analysis Observations confirmed that amorphous, crystalline and semi crystalline nature of pure PMMA, pure PEO and PMMA-PEO-LiClO4 polymer blend electrolyte thin films the SEM micrographs suggest that the surface morphology of pure PEO changes from smooth to rough when PMMA and LiClO4 added to PEO polymer, which shows the interaction/ interface between the two polymers and polymer blend electrolyte due to cross – linking. Glass transition (Tg) and melting temperatures (Tm) of pure PMMA, pure PEO and PMMA-PEO-LiClO4 polymer blend electrolyte thin films were confirmed by DSC analysis. FTIR spectra confirmed that complex formation and interaction among PMMA, PEO polymers and LiClO4 salt.
KEYWORDS:
Amorphous nature; Crystalline nature; LiClO4; PMMA; PEO
Copy the following to cite this article:
Reddy M. R, Reddy M. J, Subrahmanyam A. R. Structural, Thermal and Optical properties of PMMA, PEO and PMMA/PEO/LiClO4 Polymer Electrolyte Blends. Mat.Sci.Res.India;14(2)
|
Copy the following to cite this URL:
Reddy M. R, Reddy M. J, Subrahmanyam A. R. Structural, Thermal and Optical properties of PMMA, PEO and PMMA/PEO/LiClO4 Polymer Electrolyte Blends. Mat.Sci.Res.India;14(2). Available from: http://www.materialsciencejournal.org/?p=6259
|
Introduction
Polymer electrolytes are becoming increasingly important because of their potential use in several electrochemical devices: “smart” windows, displays, sensors, and, more importantly, rechargeable solid-state lithium batteries.1 Most of the polymer blends are immiscible and shows morphological structure. The morphology of polymer blends change the final properties of polymers. Over the past few decades, polymers like PEO, PMMA, PAN, PVA, PVDF, etc.. have been widely investigated. Among these, the PEO shows flexible chain structure and considerable conductivity at room temperature. However, low strength and low modulus limit the use of PEO. PMMA is one of the most widely studied polymer due to it having approximately 96% amorphous content at ambient temperature and also due to its promising mechanical and chemicophysical properties.2 The polymer blending method is one of the suitable approaches in overcoming this limitation. Therefore, instead of using a single polymer, blended PMMA and PEO polymers provides the required valuable properties. In addition to this blend, polymer electrolytes like Na+, Li +, Ag +, Mg +, etc.. also help to increase ionic transport in the blends. Li salt-based systems are most studied because Li+ ion is the most electropositive, which gives up electrons to form a positive Li+ with small ionic radii (0.6 Å), which can easily find path for transportation in polymer matrix.3 In the present study, LiClO4 salt is added to PMMA and PEO polymer blend to carryout structural, thermal and optical properties.
Materials and Methods
Poly(methyl methacrylate) (PMMA)(M.W~15000), poly(ethylene oxide) PEO(M. W. 2× 105) and LiClO4 salt(purity 96%) were purchased from Sigma Aldrich. The polymer blend thin films were prepared using the solution casting technique. Thin films of pure PMMA and pure PEO were prepared separately using THF (Tetra Hydro Furan) as solvent. Appropriate amount of PMMA, PEO and LiClO4 salt were separately dissolved together in THF (Tetra Hydro Furan) and stirred by using a magnetic stirrer for 24-36 hours at room temperature. Later obtained homogeneous solution was poured in a glass Petridis and then left to evaporate solvent at room temperature for several days to obtain dried blend films. The obtained films stored in a desiccator to complete dryness before characterization studies. The thickness of dried pure PMMA, pure PEO and polymer electrolyte blend thin films are around 1mm.
Table 1: Compositions of PMMA, PEO and PMMA/PEO/LiClO4 blend prepared
|
Sample code
|
wt.%
|
|
PMMA
|
PEO
|
LiClO4
|
|
A
|
100
|
0
|
0
|
|
B
|
0
|
100
|
0
|
|
C
|
90
|
10
|
0
|
|
D
|
90
|
10
|
10
|
The XRD patterns of the polymer thin films were recorded using an X-ray Diffractometer. The diffraction data were taken at room temperature with the Bragg’s angles (2θ) varying from 10 to 80 degrees. SEM micrographs of pure PMMA, pure PEO and PMMA/ PEO/LiClO4 blend films were taken by using the ZEISS instrument. The differential scanning calorimetry (DSC) of the different polymer electrolyte blend thin films were carried out using DSCQ20 instrument. About 12 mg of each sample was heated for the temperature range varying from 00C to 2000C at a heating rate of 100C.min-1. FTIR Spectroscopy measurements of pure PMMA, pure PEO and PMMA/PEO/LiClO4 blend films were recorded with the help of Spectrophotometer-Series II.
Results and Discussion
XRD
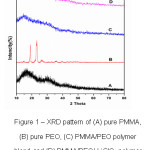
X-ray diffraction studies were performed for the structural elucidation of the polymer electrolytes.4 The XRD patterns of PMMA, PEO and their blend films are shown in Fig. 1. Pure PMMA has broad peak and Bragg’s angle were observed at 2θ=16.580 and 30.230 which indicates PMMA exhibit complete amorphous nature, on the other hand in pure PEO sharp crystalline peaks were observed at 2θ=18.860 and 23.170 which confirms complete crystalline nature of PEO. The intensity of peaks is reduced when 10 wt. % of PEO added to PMMA which indicates the disturbed crystalline nature of PEO and increased amorphous phase of PMMA (Fig.1(C)). The sharp crystalline peaks were found to be absent and broadened at 10 wt. % of LiClO4 and PEO blended with PMMA (Fig.1(D)) which indicates the semi crystalline nature of polymer electrolyte blend film this may be due to structural reorganization of polymers. A complete dissolution of lithium salt in the polymer blends is also observed.
Figure 1: XRD pattern of (A) pure PMMA, (B) pure PEO, (C) PMMA/PEO polymer blend and (D) PMMA/PEO/ LiClO4 polymer blend electrolyte films
SEM
Scanning Electron Micrograph (SEM) is generally used to study the compatibility among various constituents of the polymer electrolytes.5 Surface SEM micrographs of PMMA, PEO and their blend films are shown in Fig.2. In pure PMMA sample, homogeneous morphology was shown (Fig. 2(A)), whereas in SEM micrograph of pure PEO, appearance of rough surface with some pores and some white spots were observed. This suggests the presence of various crystalline domains (Fig. 2(B)). The surface morphology at 10 wt. % PEO in PMMA is appeared to be smooth matrix of the blend film (See Fig. 2(C)), which is the evidence of satisfactory miscibility between the two polymer matrixes.6 By adding 10 wt. % of LiClO4 salt to PMMA+PEO (90:10) blend polymer, surface morphology of electrolyte blend film becomes smooth to rough. This indicates amorphous morphology changes to semi crystalline and which increases ionic conductivity, and depends on the segmental motion of the blended polymers and solvated carrier ions.
Figure 2: SEM images of (A) pure PMMA, (B) pure PEO, (C) PMMA/PEO polymer blend and (D) PMMA/PEO/ LiClO4 polymer blend electrolyte films
FTIR
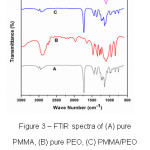
Infrared spectroscopy has been proven a highly effective means of investigating specific interactions between polymers. This tool can be used to study the mechanism of interpolymer miscibility through the formation of hydrogen bonding both qualitatively and quantitatively.7 FTIR is also a useful technique to characterize the organic, inorganic and composite materials.7 FTIR spectra of PMMA, PEO, PMMA+PEO (90:10) and PMMA+PEO+LiClO4 (90:10:10) blend films are shown in Fig.3. PMMA polymer thin film shows IR bands at 2935 cm-1 (C-H stretching band), 1726 cm-1 (C-H and O-H bending), 1444 cm-1, 1357 cm-1 (C-C stretching),1152 cm-1 (C-O stretching), 850 cm-1 and 743 cm-1 (C-C and C-O bending) (Fig.3(A)) and PEO polymer thin film shows IR bands at 2895 cm-1 (C-H stretching band), 1970 cm-1 (C-H and O-H bending), 1639 cm-1, 1463 cm-1 (C-C stretching), 1269 cm-1 (C-O stretching), 947 cm-1 and 841 cm-1 (C-C and C-O bending) (Fig.3(B)). It is observed that there is no structural change in the blend film of PMMA and PEO (Fig.3(C)) because no peaks are shifted and gets disappeared. The C-O stretching band (1152 cm-1) of PMMA is broadened at 10 wt. % of PEO and LiClO4 is blended with PMMA. The intensity of C-H and O-H bending peak (1726 cm-1) decreases and it is also observed that some peaks disappear, some are shifted towards lower wave number and some new peaks appear in the complexes indicating that there is a complex formation and interaction among PMMA, PEO polymers and LiClO4 salt.
Figure 3: FTIR spectra of (A) pure PMMA, (B) pure PEO, (C) PMMA/PEO polymer blend and (D) PMMA/PEO/ LiClO4 polymer blend electrolyte films
DSC
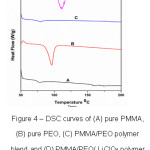
Fig.4 shows DSC results (heat flow vs. temperature) of the prepared polymer thin films of PMMA, PEO, PMMA+PEO (90:10) and PMMA+PEO+LiClO4 (90:10:10). Fig. 4(A) shows that whereas crystalline PEO exhibits a sharp melting peak, pure PMMA and PMMA/PEO blend do not. The melting temperature(Tm) of pure PMMA and pure PEO are 1580C and 950C respectively. It is observed that melting temperature(Tm) increases and glass transition temperature (Tg) decreases When 10 wt. % of LiClO4 salt added to PMMA and PEO polymer blend (Fig.4(D)). This observation suggests that the crystallinity and ionic conductivity increases because of salt presentation in the polymer blend of PMMA and PEO.
Figure 4: DSC curves of (A) pure PMMA, (B) pure PEO, (C) PMMA/PEO polymer blend and (D) PMMA/PEO/ LiClO4 polymer blend electrolyte films
Conclusions
Amorphous, crystalline and semi crystalline nature of pure PMMA, pure PEO and PMMA-PEO-LiClO4 polymer blend electrolyte thin films have been confirmed by XRD analysis. The SEM images indicate that the interaction/ interface between the polymers and LiClO4 salt due to cross–linking. Glass transition (Tg) and melting temperatures (Tm) of polymer blend electrolyte thin films were confirmed by DSC analysis. FTIR spectra confirmed that complex formation and interaction among PMMA, PEO polymers and LiClO4 salt.
Acknowledgements
The authors would like to thank Head, BOS, Department of physics, Osmania University and JNTUH for their encouragement and one of the authors MRR wishes to thank the Principal, Head(S&H), MVSR Engineering college for their constant support to carry out this work.
References
- Khan S. A., Baker G. L and Colson S. S., Chem. Mater. 1994;6:12.
- Shukla N, Thakur AK. Role of salt concentration on conductivity optimization and structural phase separation in a solid polymer electrolyte based on PMMA-LiClO4. Ionics. 2009;15:357–367.
CrossRef
- Sharma P & Kanchan D. K. Ionics. 2013;19:1285–1290.
CrossRef
- Flora X. H., Ulaganathan M and Rajendran S. Int. J. Electrochem. Sci. 2012;7:7451-7462.
- Sharma P., Kanchan D. K andGondaliya N. Open Journal of Organic Polymer Materials. 2012;2:38-44.
CrossRef
- Reddy R. M. Materials Today. Proceedings. 2016;3:3713–3718.
- Kuo W. S and Chang C. F. Macromolecules. 2001;34:4089-4097.
CrossRef
- Pradhan D. K., Choudhary R. N. P., Samantaray B. K., Thakur A. K and Katiya R. S. Effect of Montmorillonite Filler on Structural and Electrical Properties of Polymer Nano compsite Electrolytes. Ionics. 2009;15(3):345-352.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , M. Jaipal Reddy 2 and A. R. Subrahmanyam1
, M. Jaipal Reddy 2 and A. R. Subrahmanyam1 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal