A Study of Photocatalytic Degradation of Betalain Pigment from Kitchen Waste, Semiconductive Nanostructured TiO2 Used as a Photocatalyst
G. Muthukumar, B .Arjunkumar, R. Vignesh, G. Ramalingam*
Quantum Materials Research Lab(QMRL), Department of Nanoscience and Technology, Alagappa University, Karaikudi 630003, Tamil Nadu, India.
Corresponding Author E-mail: ramanloyola@gmail.com
DOI : http://dx.doi.org/10.13005/msri/150310
Article Publishing History
Article Received on : 8-Dec-2018
Article Accepted on : 16-Dec-2018
Article Published : 19 Dec 2018
Plagiarism Check: Yes
Reviewed by: Prashant K
Second Review by: Pacuru.Mohan Babu
Final Approval by:
Jit Satyabrata
Article Metrics
ABSTRACT:
Well crystallinenanostructured TiO2 powder was synthesised by Co-precipitation method using Titanium (IV) isopropoxide and CTAB are precursor materialsto maintain in 1:1 ratio concentration. The synthesised power was structurally, morphologically and optically analysed to using X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and UV-Vis absorption spectra. The betalain pigment of polluted water was prepared from Beta vulgaris extract at room temperature (RT). The 89% of betalain pigment was degraded at 40 min from Beta vulgaris extract polluted water using as-prepared TiO2 powder as a photocatalystmaterial under visible light.
Copy the following to cite this article:
Muthukumar G, Arjunkumar B, Vignesh R, Ramalingam G. A Study of Photocatalytic Degradation of Betalain Pigment from Kitchen Waste, Semiconductive Nanostructured TiO2 Used as a Photocatalyst. Mat.Sci.Res.India;15(3).
|
Copy the following to cite this URL:
Muthukumar G, Arjunkumar B, Vignesh R, Ramalingam G. A Study of Photocatalytic Degradation of Betalain Pigment from Kitchen Waste, Semiconductive Nanostructured TiO2 Used as a Photocatalyst. Mat.Sci.Res.India;15(3). Available from: http://www.materialsciencejournal.org/?p=12511
|
Introduction
In recent years, the natural and synthetic pollutant has been increasing in modern development countries in the world.1-2 The many researches was done to monitored and control the synthetic pollutant such as textiles dye3 and pharmaceuticals,4 but still natural polluted wastewaters such as sewage water, kitchen waste etc. are under in rest, so more research is wanted to control the penetration of natural pollutant contain some organic compounds into the environment.
The TiO2 is the best photocatalytic material under UV light with wavelength less than 390 nm from the literature. It is the largely commercially available, low cost, non-toxic, more thermal and chemical stability,5 So most of the researcher gives attention to TiO2 for solar cell fabrications, gas sensor and degradation of textile dyes due to low cost and user-friendly.6-8
Antholyanin, chlorophyll, carotenoid carotene and xanthophyll are the naturally available pigment presented in varies plants.9-10 Among them, Betalain is the interesting pigment commercially available sources in leaves, fruits, plants and it occurs in fungie (e.g Fly agaric). The betalain are easily water soluble nitrogen containing pigment and betalamic acid is the chromosphere of betalain pigment. It divided into two types structurally red-violet betacyanins with maximum absorption in at λmax~535 nm and yellow-orange betaxantins with maximum absorption at λmax~480 nm. The pH range from 3 to 7 the betalain have in stable condition and pH value less than 3.5, the maximum absorption peak shift to lower wavelength side.11 The Beta vulgaris (Beta vulgaris L.) is the crop commercially available easily to get betalain pigment. The major content of Beta vulgaris is betalain of 75-95% of the red pigment.12-13
In commercially, betalain pigment is used for food dye exactly, in colour changing purpose because it is cheaper, easy availability and there is no notable side-effect. Some examples of usage of betalain pigment in ice-cream, sweet, dry mixes, confectioneries, jams, candy and desserts.13-14
The degradation kinetic of color change in Beta vulgaris puree was studied with respect to temperature range of 50-120 ˚C.12 The photocatalytic degradation of levofloxacin was done under UV light to achieved 90% degradation in 120 min using TiO2 nanoparticles as a photocatalytic material.15 The photocatalytic degradation of wattle (Acacia mearnii) extract under visible light using mixed catalyst CdWO4-ZnO powder.16 The wastewater of synthetic apple juice was degraded under solar light.17
In this paper, nanostructured TiO2 powder was synthesised by Co-precipitation method and to degraded kitchen wastewater (betalain pigment). The prepared samples are characterised by XRD, SEM and UV-Vis absorption studies. The prepared nanostructured TiO2powder was used as a photocatalyst to degrade the betalain pigment presented in wastewater under visible light at RT.
Experimental Section
Preparation of Nanostructured TiO2powder
Thenanostructured TiO2powder was synthesised by Co-precipitation method as follow the literature.18 Titanium (IV) isopropoxide (Ti[OCH(CH3)2]4, 97%) and Cetyl-N,N,N – trimethylammonium bromide (CTAB) was used as a precursor. In this synthesis process, 25 ml of deionised water was mixed into 6.25 ml of ethanol (C2H5OH, 99.5%), add 0.91 g of CTAB and stirred for 30 min. 3.58 ml of Titanium (IV) isopropoxide was added and constant stirring for 24 h. The prepared milky solution was aged at 24 h. The precipitate was centrifuged several times with water, finally washed with ethanol and annealed at 80 °C for 12 h in hot air oven. The resultant amorphous TiO2 powder was calcined at 450 °C for 4 h and finally to obtained nanostructured TiO2 powder.
Photocatalytic Degradation Activity
Photocatalytic degradation of betalain in aqueous solution under visible light of 10 W LED bulb. Fresh Beta vulgaris was purchased from local market and washed it in deionised water, to remove the water soluble solvent and dust particles. To pealed out the skin of Beta vulgaris and scrapped into small pieces. 4 g of sliced Beta vulgaris in 100 ml of water and stirred it well in dark place for 10 min. The prepared natural betalain pigment was filtered and pH of the wastewater was 5.8 in RT. 5 mg of prepared TiO2 nanoparticles was added it in prepared betalain pigmentin polluted water and continuous stirring under visible light. The entire reaction was take place into manually made photocatalytic chamber. The photocatalytic degradation was continuously studied by UV-Vis absorption spectra.
Characterisation Measurement
The structural, phase and crystallite size of the as-prepared nanostructured TiO2 powder was studied by X-ray Diffraction pattern (PANalyticalX’pert Pro) using Cu-Kradiations with wavelength λ=0.15406 nm in 2 range from 20˚ to 80˚. The morphological study of synthesised TiO2powder was investigated by SEM (VEGA 3 TESCAN). The optical properties of the synthesised powder was analysed by UV-Vis spectrophotometer (Elico, SL-159). The manually made photocatalytic chamber was used to study the photocatalytic degradation activity of betalain pigment in wastewater under visible light (10W, NEO+ LED,Surya and India).
Result and Discussion
XRD Studies
Figure 1: XRD image of synthesised nanostructured TiO2 powder.
The crystallinity of synthesised nanostructured TiO2 powder was analysed by X-ray diffraction (XRD) pattern as shown in Fig. 1. From this result, the diffraction peaks are presented at 25.5˚, 38.0˚, 48.1˚, 54.2˚, 55.3˚, 63.0˚, 69.1˚, 70.7˚ and 75.3˚ corresponding to the plane of indexed to (101), (004), (200), (105), (211), (204), (116), (220) and (215). The synthesised powder have anatase system, tetragonal structure with body-centred lattice and its pattern matches JCPDS file (Card No: 89-4921).19-20 The crystallite size (D) of material was founded by using scherrer’s Formula21 are given below.
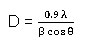
Where λ is the X-ray Wavelength of 1.5406 Å, β is full with half maximum of the peak (in radians) and = Bragg diffraction angle (˚). The average crystallite size of nanostructured TiO2 powder was about 16 nm roughly. And the crystallinity of XRD figure was also confirmed the purity of TiO2 composite.
Figure 2: SEM micrograph of nanostructured TiO2 powder (a) 2µm (b) 1 µm .
Figure 3: UV- Vis absorption spectra of (a) Nanostructured TiO2 powder,(b) Tauc’s plot of synthesised TiO2 powder (c) Betalain pigment in water.
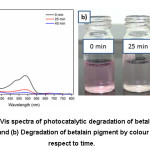
Figure 4:(a) UV-Vis spectra of photocatalytic degradation of betalain pigment in polluted water and (b) Degradation of betalain pigment by colour changes with respect to time.
SEM Analysis
The size and distribution of synthesised nanostructured TiO2 powder with different magnification was studied by using SEM micrograph as shown in the fig. 2 (a&b). From this SEM graph, agglomerated TiO2 particles are found in nanometer size and average agglomerated particles size was approximately less than 90 nm, it was roughly confirmed using SEM particle size mapping.. As represented in Fig. 2a&b, meso-TiO2 is made of large irregular shaped particles formed due to aggregation of several uneven spherical nanograins. The surface of film exhibits a porous structure (Fig. 2). It may be realized that further heat treatment of the film at 450 0C and above results may improving inter-particle contacts thereby enhancing the transport of photogenerated electrons.18
Optical Properties
UV-Vis absorption spectrum of synthesised TiO2 powder was carried out by using UV-Visspectrophotometer in the range between 200 to 900 nm as shown in the Fig. 3 a. It gives, the synthesised powder have more active in ultraviolet region. Fig.3 b shows the Tauc’s plot which is used to find the optical band gap (Eg) of the synthesised nanostructured TiO2. The calculated band gap is found 3.16 eV. The band gap value of this sample is smaller to bulk TiO2 (3.2 eV) from the report22 and it confirms that quantum confinement effect in the material. The absorption spectrum of betalain pigment extracted from Beta vulgaris was shown in the fig. 3 c. In this spectra exhibit an absorption peak around at 535.74 and 482 nm corresponding to red-violet betacyanins and yellow-orange betaxantins presented in the betalain pigment in literature.11 Yellow-orange betaxantins was presented higher in the prepared natural pigment.
Photocatalytic Degradation Activity
The photocatalytic activity of synthesised nanostructured TiO2 powder was studied by using UV-Vis absorption spectrum using under visible light as in fig. 4 a. The as-prepared TiO2 powder was added in betalain pigment polluted water with continuous stirring under visible light in room temperature. The betalain pigment was degraded continuously depends upon time. The colour monitoring experimental image of betalain pigment degradation with respect to time as shown in Fig. 4 b. The 60% of degradation was reached at 25 min and then, 40 min later 89% of degradation was achieved.
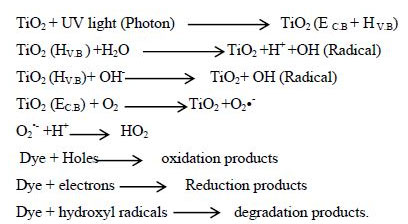
The photo degradation under UV-visible light source involves absorption of a photon by the photocatalyst which causes charge separation and excites electron to conduction band of the catalyst resulting in generation of active hydroxyl radicals by oxidation of water molecules with photo generated holes and oxygenated species that attack the dye molecules. The generated electrons by photon absorption either reacts with O2 adsorbed on titanium surface or reacts with water to give superoxide anion radicals or it may reduces the dye. The holes are responsible for generation of OH radicals, by reacting with hydroxylions or water. The hydroxyl radical exists for nanoseconds, thus when they formed they immediately reacts.23-26 The important reactions which takes place on titania surface for degradation of organic dyes are.
Conclusion
Nanostructured TiO2 powder was successfully synthesised by co-precipitation method at room temperature and worked as a photocatalytic material to degrade betalain pigment. The structural, morphological and optical properties of the synthesised TiO2 powder were done. In photocatalytic degradation process, 89% of betalain pigment was degraded in polluted water at 40 min under visible light. The degradation conditions strongly affect the nanostructure of TiO2 and The photocatalytic degradation of organic dyes is largely dependent on morphology of TiO2 nanostructures. The TiO2 nanopowder can be easily recycled and reused having good stability and photocatalytic activity. We expect that TiO2 nanopowder with different morphologies is favourable and good photo catalysts for treating dye effluents and industrial wastewater and kitchen waste.
Acknowledgements and Funding Source
This work was fully supported by the Alagappa University Research Fund-2017(AURF) and instrumentation facility has been used under DST-SERB (File no:EEQ/2016/198) Govt.of India project.
Conflict of Interest
“The author(s) declare(s) that there is no conflict of interests regarding the publication of this article
References
- A.Agarwal. Water pollutions posed by small industries: a case study of India and china. Water Science and Technology. 2002;45(8):47-52.
CrossRef
- J. Fu, B. Mai, G. Sheng, G. Zhang, X. Wang, P. Peng, X. Xiao, R. Ran, F. Cheng, X. Peng, Z. Wang, U. W.Tang. Persistent organic pollutants in environment of the Pearl River delta, china: an overview. Chemosphere. 2003;52:1411-1422.
CrossRef
- R. Saravanan, S. Karthikeyan, V.K. Gupta, G. Sekaran, V. Narayanan, A. Stephen. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination.Materials Science and Engineering C. 2013;33:91-98.
CrossRef
- K.Ikehata, N. J.Naghashkar, M. G. El-Din. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone: Science and Engineering. 2006;28:353-414.
CrossRef
- A.R. Khataeea, M.B. Kasiri. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes.Journal of Molecular Catalaysis A: Chemical. 2010;328:8-26.
CrossRef
- Z. S.Wang, H.Kawauchi, T. Kashima, H. Arakawa. Significant influence of TiO2photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coordination Chemistry Reviews. 2004;248:1381-1389.
CrossRef
- R. Rella, J. Spadavecchia, M.G. Manera, S. Capone, A. Taurino, M. Martino, A.P. Caricato, T. Tunno. Acetone and ethanol solid-state gas sensors based on TiO2 nanoparticles thin film deposited by matrix assisted pulsed laser evaporation.Sensors and Actuators B. 2007;127:426-431.
CrossRef
- C.C. Chena, C.S. Lu, Y.C. Chung, J.L. Jan. UV light induced photodegradation of malachite green on TiO2 nanoparticles.Journal of Hazardous Materials. 2007;141:520-528.
CrossRef
- F. Delgado-Vargas, A. R. Jiménez, O. Paredes-López. Natural pigments: carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Critical Reviews in Food Science and Nutrition. 2000;40(3):173-289.
CrossRef
- I. C.Maurya, Neetu, G. Arun Kumar, P. Srivastava, L. Bahadur. Natural dye extracted from saracaasoca flowers as sensitizer for TiO2-based dye-sensitized solar cell.Journal of Solar Energy Engineering. 2016;138: 051006.
CrossRef
- K. U. Isah, U.Ahmadu, A. Idris, M.IsahKimpa, U. E. Uno, M. M.Ndamitso, N.Alu. Betalain pigments as natural photosensitizers for dye-sensitized solar cells: the effect of dye pH on the photoelectric parameters.Mater Renew Sustain Energy. 2015;4:39.
CrossRef
- J.Chandran, P.Nisha, R. S. Singhal, B. Anirudha,Pandit. Degradation of color in Beta vulgaris (Beta vulgaris L.): a kinetics study.Journal of Food Science and Technology. 2014;51(10):2678-2684.
CrossRef
- V. K. koul, M.P. Jain, S.Koul, V. K. Sharma, C. L. Tikoo, S. M. Jain. Spray drying of beet root juice using different carries.Industrial Journal Chemistry Technology. 2002;9:442-445.
- R. Kakali, G. Swathi, U. R. Chaudhuri, R. Chakraborty. The use of a natural colorant based on betalain in the manufacture of sweet products in India.International of Food Science and Technology. 2004;39:1087-1091.
CrossRef
- S. K.Kansal, P.Kundu, S.Sood, R.Lamba, A. Umar, S.K.Mehta. Photocatalytic degradation of antibiotic levofloxacin using well-crystalline TiO2 nanoparticles.New Journal of Chemistry. 2014;38(7):3220-3226.
CrossRef
- E. T. Deva Kumar, K. Thirumalai, R. Aravindhan, M. Swaminathan, J. Raghava Rao, B. U. Nair. Visible light photocatalytic degradation of wattle extract of mixing CdWO4 over semiconductiveZnOphotocatalyst. Royal Society of Chemistry Advances. 2015;5(75):60926-60937.
- A. Durán, J.M.Monteagudo, A.Carnicer, I.SanMartín, P.Serna.photodegradation of synthetic apple juice wastewater: process optimization and operational cost study. Solar Energy Materials & Solar Cells. 2012;107:307-315.
CrossRef
- R. G. Satyanarayana, K.Ananthanarayanan, C. Yap, M.Grätzel, P. Balaya. Synthesis of mesoporous titanium dioxide by soft template based approach: characterization and application in dye-sensitized solar cells. Energy & Environmental Science. 2013;3:838-845.
- A. Lamberti, A. Sacco, D. Hidalgo, S. Bianco, D. Manfredi, M. Quaglio, E. Tresso, C.F. Pirri. TiO2 nanotube array as efficient transparent photoanode in dye-sensitized solar cell with high electron lifetime, ActaPhysicaPolonica Series a. 2013;123(2):376-379.
CrossRef
- A.Arivarasan, S. Bharathi, V. Vijayaraj, G.Sasikala, R. Jayavel. Evaluation of reaction parameters dependent optical properties, J InorgOrganometPolym. 2018;23(3):1263-1275.
- JacekWojnarowicz, A. Opalinska, T.Chudoba, S.Gierlotka, R. Mukhovskyi, E. Pietrzykowska, K.Sobczak, W.Lojkowski. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis.Journal of Nanomaterials. 2016;2789871.
- K. M.Reddya, S. V. Manoramaa, A. Ramachandra Reddy. Bandgap studies on anatase titanium dioxide nanoparticles, Materials Chemistry and Physics. 2002;78:239-245.
- S. B. Khana, M.Houa, S. Shuanga, Z. Zhang. Morphological Influence of TiO2 Nanostructures (nanozigzag, nanohelics andnanorod) on Photocatalytic Degradation of organic dyes. Applied Surface Science. 2017;400:184-193.
CrossRef
- M. E. Borges, M. Sierra, E. Cuevas, R. D.García, P. Esparza. Photocatalysis with solar energy: Sunlight-responsive photocatalyst based on TiO2 loaded on a natural material for wastewater treatment. Solar Energy. 2016;135:527-535.
CroeeRef
- S. A. Mahdy, W. H. Mohammed, H. Emad, H. A. Kareem, R. Shamel, S. Mahdi.The antibacterial activity of TiO2 Nanoparticle, Journal of Babylon University /Pure and Applied Sciences. 2017;25(3):955-961.
- W Z Khan, I. Najeeb, S Ishtiaque. Photocatalytic Degradation of a Real Textile Wastewater using Titanium Dioxide, Zinc Oxide and Hydrogen Peroxide. The International Journal Of Engineering And Science. 2016;5(7):61-70.

This work is licensed under a Creative Commons Attribution 4.0 International License.

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal