Raghu Patel G. Ranganagowda1 , Sakshi Shantharam Kamath2
, Sakshi Shantharam Kamath2 , And Basavaraju Bennehalli3*
, And Basavaraju Bennehalli3*
1Research Scholar, Department of Chemistry, Alva’s Institute of Engineering and Technology, Visvesvaraya Technological University, MIJAR-574225, Karnataka, India
2Assistant Professor, Department of Chemistry, Alva’s Institute of Engineering and Technology, Visvesvaraya Technological University, MIJAR-574225, Karnataka, India
3Professor, Department of Chemistry, Alva’s Institute of Engineering and Technology, Visvesvaraya Technological University, MIJAR-574225, Karnataka, India
Corresponding Author E-mail: basavaraju@aiet.org.in
DOI : http://dx.doi.org/10.13005/msri/160112
Article Publishing History
Article Received on : 20-Mar-2019
Article Accepted on : 24-Apr-2019
Article Published : 25 Apr 2019
Plagiarism Check: Yes
Reviewed by: Raja Manickam
Second Review by: Kotari Rao
Final Approval by: Jit Satyabrata,
Dr. Ramachandra Naik
Article Metrics
ABSTRACT:
In areca empty fruit bunch, fibres are packed strongly with hemicelluloses, lignin and with slight deposition of wax and inorganic elements. In the work reported, for the extraction of cellulose from the raw areca fibres, formic acid (20% v/v) and hydrogen peroxide (10% v/v) were used and the yield of 65% cellulose was attained. To know α-cellulose content with crystallinity, XRD diffractions studies were carried and the values were found to be 93% and 71% respectively. FTIR spectral studies confirm the absence of hemicellulose, lignin and wax in the cellulose extracted from areca fibres. The morphological studies provided the evidence for isolated fibres and removal of deposits in the extracted cellulose.
KEYWORDS:
Areca Fiber; Delignification; Fiber Cellulose; Formic Acid; Hydrogen Peroxide
Copy the following to cite this article:
Ranganagowda R. P. G, Kamath S. S, Bennehalli B. Extraction and characterization of cellulose from natural areca fiber. Mat. Sci. Res. India; 16 (1).
|
Copy the following to cite this URL:
Ranganagowda R. P. G, Kamath S. S, Bennehalli B. Extraction and characterization of cellulose from natural areca fiber. Mat. Sci. Res. India;16 (1). Available from: https://bit.ly/2DxEopt
|
Introduction
The cellulose is commonly used in paper, film, textiles, building material and for the production of food additives. Recent researches are also focusing on solving environmental problems such as designing a cellulose-based absorbent for oil spills and heavy metal pollution on water or land1 and for making filters for industrial2 and municipal wastewater treatment.3
Natural plant fibres are made up of polymers namely cellulose, hemicelluloses and lignin and a small number of extractives. Hydrophilic cellulose is the main substance that makes up plant cell wall and contributes to the physical stability of the cells.4 Hence, cellulose is the most abundantly available organic compound on earth. Cellulose does not dissolve in water and has high crystallinity and high molecular weight.5
Cellulose is a linear polymer consisting of glucose monomer units connected through 1-4 β-linkages. The terminal reducing and non-reducing sugar units stabilized the cellulose polymer chain.6 The reactive hydroxyl groups at C-2, C-3, and C-6positions are responsible for the physicochemical properties of the cellulose polymer. The stronger intermolecular and intramolecular H-bonding, weak Van der Waals force and twisting of monomer units randomly makes the polymer chain compact and tight.7
Based on the formic acid process, cellulose was extracted from the jute fibres with 59.8% yield. The results showed that the jute fibres have 10.9% lignin content. The acid hydrolysate of cellulose contained 2.7% glucose and 0.2% xylose. In this study, it was demonstrated that 76% of hemicelluloses and 85.8% of lignin in jute could be extracted.8 Also, formic acid (20%) and hydrogen peroxide (10%) were used in the extraction of cellulose (60%) with α-cellulose (93.7%) with 70% crystallinity from oil palm empty fruit bunches (OPEFB).9 Studies using FTIR, XRD and TGA were also validated for OPEFB and commercially available microcrystalline cellulose (MCC).
The alkali and bleaching treatments on fibres extracted from oil palm fronds succeeded in extracting cellulose with 40% yield on a dry weight basis. X-ray diffraction results exhibited a crystallinity index of 24.31% for the raw oil palm fronds, which progressively increased after alkali treatment (52.46%) and bleaching treatment (68.75%).10
Quality of cellulose fibers were studied by comparing the effects of alkali treatment and acid treatment on Calotropisprocera. NaOH (0.5N) treated C. proceragave approximately 6% of cellulose fibers. Cellulose extraction was increased with 26% efficiency, when fibers were treated with a mixture of 80% acetic acid and concentrated nitric acid in the ratio 10:1 (v/v). Infrared spectroscopy and acid detergent fiber methods were adopted to determine percentage composition of cellulose. 85% of cellulose content was observed in acid treated fibers as compared to alkali treated fibers.11
Acid hydrolysis, chlorination, alkaline extraction and bleaching of petioles of leaves (Calathealutea) [widely distributed species in Tabasco, state of México] fibers gave a yield of 26.25% cellulose. Approximately 38.09% crystallinity of the cellulose fibers and a crystal size of 2.6 Å were also observed from X-ray diffraction patterns of Calathealutea.12
Acacia leucophloeafibers (ALFs) treated with 5% (w/v) sodium hydroxide solution with 45 min soaking time have shown high cellulose (76.69wt%), low hemicellulose (3.81wt%), lignin (13.67 wt%) contents and it is evident that, the results of chemical and X-ray diffraction exhibit higher crystallinity index (74.27%).13
Cellulose, hemicellulose and lignin contents ofFicus (Peepal tree) leaf fibers were studied for both untreated and alkali pretreated at 30oC maintaining a liquor ratio of 30:1. The hemicellulose content for the pretreated fibers decreased from 30.5% to 12.6%, the α-cellulose and lignin content increased from 38.1% to 46.8% and 23.4% to 36.5% respectively. The α-cellulose content in extracted cellulose was found to be increasing after various chemical treatments from 46.8% to 90.6% in comparison with the untreatedfibers.14
In India, areca nut is an important commercial crop grown in about 51,00,000 hectares in Andhra Pradesh, Assam, Meghalaya, Tripura, Mizoram, Andaman and Nicobar Islands, Maharashtra, Goa, Karnataka, Kerala, Tamil Nadu, West Bengal and Pondicherry.15 This provides an enormous amount of sustainable and biodegradable husk for many value-added products. Areca husk is a lignocellulosic material comprised of cellulose with varying proportions of hemicelluloses (35–64.8%), lignin (13.0–26.0%), pectin and protopectin16out of which about 60% can be changed into value added biopolymer.17 To summarize, cellulose is a naturally available biopolymer and is considered as a good replacement for conventional polymers.18 Cellulose would be extracted from plant fibers using some of the chemical and mechanical methods and they could be extracted in nano and micro forms by alkalization, bleaching and acid hydrolysis process.19-23
The extraction of cellulose from areca husk using an inexpensive method would serve as an alternative environmental friendly way of managing the largely produced areca residue. The purpose of this study was to extract cellulose from areca husk using a cost-effective method and to characterize it.
Materials and Methods
Fibre Extraction
Areca empty fruits were collected from Alva’s Farm House, Mijar, Karnataka, India and were immersed in double distilled water for a period of 5 days; permitting the fibre to be extracted from the empty fruit effectively. The extracted areca fibres were maintained at a temperature of 30oC and relative humidity of 70% for a period of 72 h. The analytical grade formic acid, sodium hydroxide, hydrogen peroxide, and ethanolwere used as received. The commercial cellulose was used as a standard.
Moisture content of areca fiber
The percentage moisture content of areca fiber was determined according to ASTM 2003 standard. 10 g of raw areca fibers werekept in a crucible and maintained at a temperature of 100±2oC for 3 h in an oven. Later, the fibers were kept in a desiccator until they attain room temperature and weighed. The reported result is an average triplicate.
Ash content of areca fiber
ASTM 2007 standard was used for the evaluation of percentage ash content of raw areca fibre in which fibres were initially maintained at 105oC for 3 h and were pulverized. A nylon filter, Kexin, 40 mesh, was used for sieving the grounded sample. 2 g of grounded sample was kept in a clean crucible and the crucible was placed in a furnace for 1 h at 600oC. Later, the crucible was taken out of the furnace and placed in a desiccator. The sample was weighed when it attained room temperature. The reported ash content of raw areca fibre is an average of triplicate.
Fibre-Cellulose Extraction
The extracted areca fibres were initially washed with 1% detergent to remove oil & grease and then dried at lab temperature and were then cut into a length of 0.2 mm. Final drying of the fibres was done at 100 ± 2 oC in an oven.
The dewaxing of raw fibres was done in a Soxhlet apparatus for 6h by using 200 mL of 70% (v/v) ethyl alcohol. The fibre to solvent ratio was 1:10 (g L-1). The collected fibres were washed thoroughly with double distilled water to remove the traces of alcohol and dried. 10g of dewaxed fibres were taken in a beaker and suspended in 100 mL of 10% sodium hydroxide, followed by 100 mL of 10% of H2O2. The beaker was covered with aluminium foil and maintained at 121 oC and 1.5 bar in an autoclave for 1h. The fibres separated from the supernatant were washed thoroughly with double distilled water.
Delignification of autoclaved fibres was carried out by soaking them in a 1:1 (v/v) mixture of 20% formic acid and 10% hydrogen peroxide. The mixture was maintained at a temperature of 85 oC for 2 h on a water bath and after filtration, dignified fibres were collected. The fibres were initially washed with 10% formic acid and repeated washings with double distilled water were carried out. The cellulose fibres extracted were light yellow in colour. It was then treated with 10% hydrogen peroxide for 90 min at 60 oC. Sodium hydroxide (10%) solution was used to adjust the pH. The white suspension obtained was filtered and washed several times. The insoluble fraction of cellulose was collected and the yield (w/w) was calculated.9,24 TAPPI method was used to calculate the α-cellulose content.25
FTIR Spectral Studies
FTIR spectrophotometer, model Perkin Elmer 2000 was used forthe analysis of possible chemical bonding existing in the raw areca fiber, fiber cellulose and the commercial cellulose. FTIR spectra were analyzed in a range between 500 cm-1 and 4000 cm-1.
Morphological Studies
The SEM images of raw areca fiber, fiber cellulose and commercial cellulose were taken by using JEOL JSMT330A scanning electron microscope at the accelerating voltage of 20 KV.
X-Ray Diffraction Studies
The phase behavior of raw areca fiber, fiber cellulose and commercial cellulose was studied by using an X-ray diffractometer. The analysis was done at 1.540 Ao wavelength (Cu-Kα radiation), with a scanning speed of 2o/s and a 2θ range of 3-80o. The crystallinity index (CIr) and The crystallite size were calculated using an equationsCIr = [(I002-Iam/I002)]×100 and D= Kλ / B cosθrespectively.
Where;
I002=The highest peak intensity of the crystalline fractions,
Iam= The low intensity peak of the amorphous region,
K = constant, 0.91,
θ = Bragg’s angle, and
β = The intensity of the full width at half of the maximum (FWHM) corresponding to a high intensity peak of the diffraction plane 002.26
Results and Discussion
Extraction of Fiber Cellulose
The moisture and ash content of raw areca fiber determined according to ASTM 2003 standard were found to be1.81% and 1.30%, respectively. The yield of cellulose from delignified fibers was 65% (w/w) on a dry weight basis. The high yield of cellulose is achieved as performic acid effectively removed the lignin and hemicellulose and thus proves that the method adopted is efficient.The high energy steam produced in an autoclave during the pretreatment process support thediffusion of sodium hydroxide and hydrogen peroxide for partial oxidation and dislocated the fibrillar arrangement.Formic acid, with its major contributors, is very stable in an aqueous medium.In case of formic acid, the explicit negative charge on the oxygen atom is destabilizing the molecule and the molecule needs to be stabilized by distributing or delocalizing it. In an aqueous medium, the destabilizing negative charge is distributed over two oxygen atoms, hence stabilizing the formic acid.Further, the multiple bond between the C=O moves onto a connecting oxygen atom and thus the connecting oxygen atom bears the negative charge. The maximum stability of formic acid is achieved by the constant backward and forward movement of electrons through single bond producing equivalent structures or major contributors.This may form performic acid by accepting an oxygen atom from hydrogen peroxide.
The non-crystalline region of cellulose is dissolved in the stabilized formic acid medium whereas crystalline region of cellulose is unaffected by the acid attack which increases the crystallinity. These synergistic effects convert hemicellulose and lignin into the corresponding water soluble salts and carboxylic acids respectively leaving behind the insoluble suspension of crystalline cellulose in aqueous medium.
The α-cellulose content of isolated fiber cellulose was determined according to TAPPI method and was found to be 92.8%.25
FTIR Spectral Studies
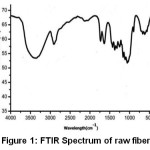
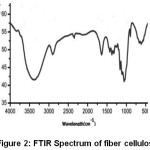
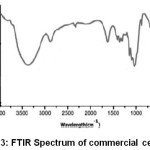
The transmittance peaks observed at 3420 cm-1 and 2954 cm-1 in the spectra of raw fiber (Figure 1), fiber cellulose (Figure 2) and commercial cellulose (Figure 3) were attributable to stretching vibrations of cellulose –OH and C-H groups respectively27.The transmittance peaks at 1463, 1377, and 1275 cm-1 corresponding to bending vibrations of -CH2, C-H, and C-O of cellulose were observed in the spectra of raw, fiber cellulose and commercial cellulose.
The water absorption peak28 was observed at 1661 cm-1 and the peak corresponding to the β-glycosidic linkage at 898 cm-1was present in all of the spectra. However, the peaks at 1727 cm-1 and 1128cm-1in the spectra of raw fiber (Figure 1) attributes to waxy C=O acetyl group of hemicellulose ester or carbonyl ester of the lignin unit and C-O-C of aryl-alkyl ether in lignin respectively were found to be absent in the spectra of fiber cellulose and the commercial cellulose (Figure2 and Figure 3).29-30 Further, peaks corresponding to deformation of the C–H rocking vibration and the C–O–C pyranose, characteristics of cellulose are more prominent Figure 2 and Figure 3 at 1161, 1137, and 1105 cm-1 demonstrates the removal of hemicelluloses and lignin.31
Figure 1: FTIR Spectrum of raw fiber
Figure 2: FTIR Spectrum of fiber cellulose
Figure 3: FTIR Spectrum of commercial cellulose
Morphology
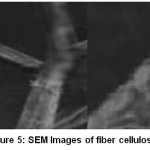
The SEM image of raw fiber confirms that areca fiber is a Lignocellulosic polymer composite with a fibrillar packing as shown in Figure 4. The image of external fiber surface reveals the irregular deposition of wax, hemicelluloses and lignin. The images of fiber cellulose Figure 5 show the separated fibrils suggest the removal of wax, hemicelluloses and lignin deposits from the fiber surface. Further, the external surface is a mixture of both smooth surface and scars due to the of inorganic particles and the metal components.
Figure 4: SEM Images of raw fiber
Figure 5: SEM Images of fiber cellulose
X-Ray Analysis
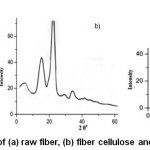
The XRD image of raw fiber presented in Figure 6 (a)suggests that raw areca fiber is a composite of hemicellulose and lignin which are amorphous and crystalline cellulose. The small peak intensity may be due to the entrapment of crystalline cellulose in amorphous hemicellulose and lignin. The high intensity peak in X-ray diffractogram of fiber cellulose Figure 6(b) at 22.6o (002) which is analogous to diffractogram of commercial cellulose Figure 6(c) confirms the hemicellulose depolymerization and delignification.The crystalline nature and the amount of crystallinity is described by the sharpness and the peak intensity value respectively. The characteristic peaks of cellulose; a high intensity crystalline peak (Iam) and amorphous peak (Iam) corresponding to the crystallographic plane (101) are exhibited in the diffractogram of fiber cellulose at 2θ=22.1 and
2θ=18o respectively.For the raw areca fiber, crystallinity index and the crystallite size were found to be 44.0% and 31.6nm, respectively and for the fiber cellulose these values were 71% and 9.6nm respectively.
Figure 6: XRD patterns of (a) raw fiber, (b) fiber cellulose and (c) commercial cellulose
Conclusions
A method developed for the extraction of cellulose from the areca fiber by using formic acid and hydrogen peroxide in lower concentration yielded 65% cellulose. The cellulose found to contain α-cellulose to an extent of 92.8% with 70% crystallinity. The FTIR spectral studies confirms the removal of wax, lignin, and hemicelluloses from the fiber surface effectively. X-Ray diffraction studies demonstrated the crystalline nature of fiber cellulose. SEM images exhibited that the cellulose extracted from areca fiber appeared as separate fibrils with crystallite parallel chains.
Acknowledgements and Funding Source
The author, Basavaraju Bennehalli is thankful to the Vision Group on Science & Technology (VGST), Department of IT, BT and Science & Technology, Government of Karnataka for financial support in the form of sanctioning a Research Project to carry out the present investigation (grant number VGST/CISEE/2012-13/282 dated March 16, 2013.
References
- S.T. Nguyen, J.B Feng, N.T. Le, A.T.T. Le, N. Hoang, V.B.C. Tan and H.M. Duong. Cellulose aerogel from paper waste for crude oil spill cleaning. Industrial and Engineering Research, 52: 18386 – 13891.
CrossRef
- Z. He, M. Meng, L. Yan, W. Zhu, F. Sun, Y. Yan, Y. Liu, And S. Liu. Fabrication of new cellulose acetate blend imprinted membrane assisted with ionic liquid ((BMIM)Cl) for selective adsorption of salicylic acid from industrial wastewater. Separation and Purification Technology, (2015); 145: 63 – 74.
CrossRef
- S. K. Nataraj, S. Roy, M.B. Patil, M.N. Nadagouda, W.E. Rudzinski, and T.M. Aminabhavi, Cellulose-acetate-coated α-alumina ceramic composite tubular membranes, for wastewater treatment. Desalination, (2011); 281: 348 – 353.
CrossRef
- B. Duchemin, A. Thuault, A. Vicente, B. Rigaud, C. Fernandez, C. and S Eve. Ultrastructure of cellulose crystallites in flax titles fibers. Cellulose, (2012); 19(6): 1837 – 1854.
CrossRef
- L. Kuutti. Cellulose, starch and their derivatives for industrial applications. VTT Technical Research Centre of Finland, Finland: (2013); pp. 35 – 37.
- D. Klemm, B. Heublein, H. P. Fink, and A. Bohn, Cellulose: Fascinating biopolymer and sustainable raw material. Angewandte Chemie International Edition (2005); 44(22), 3358-3393.
CrossRef
- M. A. Abdullah, M. S. Nazir, and B. A. Wahjoedi, Development of value- added biomaterials from Oil Palm Agro-wastes. 2nd International Conference on Biotechnology and Food Sciences (ICBFS), Bali, Indonesia. (2011).
- M. Jahan, A. Saeed, Z. He, and Y. Ni. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose (2011); 18(2): 451-459.
CrossRef
- Muhammad ShahidNazir, Bambang Ari Wahjoedi, Abdul Wahid Yussof, and Mohd Azmuddin Abdullah Eco-Friendly Extraction and Characterization of Cellulose from Oil Palm Empty Fruit Bunches. BioResources (2013); 8(2): 2161-2172.
CrossRef
- SitiRasilaAinaaMohdRasli, Ishak Ahmad, Azwan Mat Lazim, and Ainon Hamzah Extraction and characterization of cellulose from agricultural residue – oil palm fronds. Malaysian Journal of Analytical Sciences, (2017); Vol 21 No 5 1065 – 1073.
CrossRef
- SomnathMaji, Rajesh Mehrotra and Sandhya Mehrotra. Extraction of high quality cellulose from the stem of Calotropisprocera. South Asian J ExpBiol; (2013); 3: 113‐118.
- Gloria Ivette Bolio-López, Genaro Cadenas-Madrigal, Lucien Veleva, Richard Falconi, Patricia de la Cruz-Burelo, Manuel Mateo Hernández-Villegas, Liliana Pelayo-Munoz. Extraction of cellulose fibers from tó leaf petioles (Calathealutea) and characterization. IJISET – International Journal of Innovative Science, Engineering & Technology, (2015); Vol. 2 Issue 4.
- V. P. Arthanarieswaran, A. Kumaraveland S. S. Saravanakumar. Characterization of New Natural Cellulosic Fiber from Acacia leucophloea Bark. International Journal of Polymer Analysis and Characterization, (2015); 20(4): 367-376.
CrossRef
- K. Obi Reddy, C. Uma Maheswari, E. Muzenda, M. Shukla and A. VaradaRajulu. Extraction and Characterization of Cellulose from Pretreated Ficus (Peepal Tree) Leaf Fibers, Journal of Natural Fibers, (2016); 13:1: 54-64.
CrossRef
- Dr. ParameshwaraNaik, Economic Analysis of coconut and arecanut production system a review. International Journal of Development Research. Vol. 06, Issue, 08, pp. 9175-9179 (2016).
- S. Dhanalakshmi, P. Ramadevi, B. Basavaraju, C.V. Srinivasa. Physical characterization of natural lignocellulosic single areca fiber. Ciência&Tecnologia dos Materiais 27 121–135 (2015)
CrossRef
- W.D Wanrosli, I. Mazlan, K.N Law, and R. Nasrullah. Influences of the operating variables of acetosolv pulping on pulp properties of oil palm frond fibers. Maderas: Ciencia y Technologia 13(2), 193-202 (2011).
CrossRef
- M. P. Menon, R. Selvakumar, P.Sureshkumar and S. Ramakrishna. Extraction and modification of cellulose nanofibers derived from biomass for environmental application, The Royal Society of Chemistry, RSC Adv.,7, 42750–42773,(2017)
CrossRef
- I.Ismail, D.Sa’adiyah, P.Rahajeng, A.Suprayitno, and R. Andiana, Extraction of cellulose microcrystalline from galam wood for biopolymer, Proceedings of the 3rd International Conference on Materials and Metallurgical Engineering and Technology (ICOMMET 2017, AIP Conf. Proc. 1945,(2017)
CrossRef
- K. Obi Reddy, C. Uma Maheswari, M.S. Dhlamini, B.M. Mothudi, V.P. Kommula, Jinming Zhang, Jun Zhang, A. VaradaRajulu, Extraction and Characterization of Cellulose Single Fibers from Native African Napier Grass, Carbohydrate Polymers (2018)
CrossRef
- K.Harini, K.Ramya, M.Sukumar, Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose, Carbohydrate Polymers (2018).
CrossRef
- Rehan Abbasi, and Vijay Baheti, Preparation of nanocellulose from jute fiber waste, J Textile Eng Fashion Technol, 4(1) 101-104, (2018)
CrossRef
- Khenblouche A., Bechki D., Gouamid M., Charradi K., Segni L., Hadjadj M., Boughali S., Extraction and characterization of cellulose microfibers from Retamaraetam stems, Polímeros, (2019); 29(1).
CrossRef
- Jahan M., Saeed A., He Z., and Y. Ni. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose (2011); 18(2): 451-459.
CrossRef
- Alpha-, beta- and gamma- cellulose in pulp in: Fibrous material and pulp testing.T 203 om 88, TAPPI Press, Vol. 1., Atlanta. (1991).
- A.L. Patterson. The Scherrer formula for X-ray particle size determination. Physical Review (1939); 56(10): 978-982.
CrossRef
- H.P.S.A. Khalil, H. Ismail, H.D. Rozman, M.N. and Ahmad. The effect of acetylation on interfacial shear strength between plant fibers and various matrices. European Polymer Journal (2001); 37(5): 1037-1045.
CrossRef
- Le Troedec, M., Sedan, D., Peyratout, C., Bonnet, J. P., Smith, A., Guinebretiere, R., Gloaguen, V., and Krausz, P. “Influence of various chemical treatments on the composition and structure of hemp fibers,” Composites Part A: Applied Science and Manufacturing (2008); 39(3): 514-522.
CrossRef
- Alemdar A., M. and Sain. Isolation and characterization of nano fibers from agricultural residues – Wheat straw and soy hulls. Bioresource Technology (2008); 99(6): 1664-1671.
CrossRef
- M.S. Nazir, B.A. Wahjoedi, A.W. Yussof, and M.A. Abdullah. Green extraction and Characterization of cellulose fibers from Oil Palm Empty Fruit Bunch. 2nd International Conference on Process Engineering and Advanced Materials (ICPEAM2012) (2012).
CrossRef
- H. Kargarzadeh, I. Ahmad, I. Abdullah, A. Dufresne, S. Zainudin, and R. Sheltami. “Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenafbast fibers, Cellulose, 10.1007/s10570-012-9684-6 (2012).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Sakshi Shantharam Kamath2
, Sakshi Shantharam Kamath2 , And Basavaraju Bennehalli3*
, And Basavaraju Bennehalli3*
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal