In Situ Graphene Oxide Reinforced Poly(urea-formaldehyde) Microencapsulation of Epoxy
Ayse Sezer Hicyilmaz1 , Ayse Celik Bedeloglu1*
, Ayse Celik Bedeloglu1*
1Department of Fiber and Polymer Engineering, Bursa Technical University, Bursa, Turkey.
Corresponding Author E-mail: ayse.bedeloglu@gmail.com, ayse.bedeloglu@btu.edu.tr
DOI : http://dx.doi.org/10.13005/msri/160103
Article Publishing History
Article Received on : 26-Mar-2019
Article Accepted on : 04-Apr-2019
Article Published : 05 Apr 2019
Plagiarism Check: Yes
Reviewed by: Dr. YOGESH BHOGE
Second Review by: R Prabu Mech
Final Approval by: Sitansu Sekhar Nanda
Article Metrics
ABSTRACT:
In this study, graphene oxide (GO) reinforced epoxy loaded poly(urea-formaldehyde) microcapsules have produced by easy and cost-effective one step in-situ emulsion polymerization method without emulsifier agent. Low amounts of GOhas incorporated into microcapsules without pre-treatment or ultra-speed agitation step. The effect of GO reinforcement on microcapsule size, shell structure, morphology, and thermal behaviour analyzed with Fourier transform infrared spectrometer (FTIR), scanning electronic microscope (SEM) and thermal gravimetric analysis (TGA), respectively. Results revealed that obtained microcapsules are resistant up to 225 °C. Besides, also, the addition of a certain amount of GO (0.5%, wt/ wt) forms pretty small sized (3-4 µm) microcapsules with a smooth surface, since GO nanoparticles act as a surfactant material. On the other hand, by increasing the amount of GO up to 1% (w / w), higher shell thickness was caused due to the coating of the GO layers into the shell. Produced GO-reinforced epoxy loaded microcapsules could be used in self-healing composites and also anticorrosion coatings.
KEYWORDS:
Anti-Corrosion; Epoxy; Graphene Oxide; Microcapsule; Poly(Urea-Formaldehyde); Self-Healing
Copy the following to cite this article:
Hicyilmaz A. S, Bedeloglu A. C. In Situ Graphene Oxide Reinforced Poly(urea-formaldehyde) Microencapsulation of Epoxy. Mat. Sci. Res. India; 16 (1).
|
Copy the following to cite this URL:
Hicyilmaz A. S, Bedeloglu A. C. In Situ Graphene Oxide Reinforced Poly(urea-formaldehyde) Microencapsulation of Epoxy. Mat. Sci. Res. India; 16 (1). Available from: https://bit.ly/2K5bJNV
|
Introduction
Self-healing materials are very important due to increased service life and decreased maintenance costs. Since composites are composed of two or more different compounds, detection of damages and repair of the damages in the composites are very difficult.1 So, especially, composites require self-healing property. Different methods are used to produce self-healing composites such as vascular systems, microcapsules or introduction of reversible bondings to the material etc. Embedding of microencapsulated healing agents in the composites is one of the most effective and applicable methods.2 When damage occurs in the composite, microcapsules rupture and the healing agent releases then fills the damaged region then, solidifies by polymerization reaction.3
In the literature, various self-healing agents have been used. Dicyclopentadiene (DCPD) have been used with Grubbs Ring-Opening Metathesis Polymerisation (ROMP) catalyst in the epoxy matrix composites.4 Epoxy compounds have been used with different curing agents in the matrix materials.5 Epoxy compounds have been used with solvents in the microcapsules to increase the healing effect by swelling of the matrix.6, 7 Also, two microcapsules system have been used in composites; epoxy loaded microcapsules and hardener loaded microcapsules embedded in the matrix.8 On the other hand, epoxy loaded microcapsules have been used in anticorrosion applications.9-11 Urea- formaldehyde system is reactive, cost-effective and frequently used monomer system to obtain shell material in the microencapsulation process.12 However, mechanical and thermal properties are not enough for harsh process conditions such as high temperature over 200 °C or high mixing rates.
Modification of microcapsule properties is possible by changing microencapsulation process conditions (stirring rate, emulsifier, core-shell ratio etc.) or surface modification with the addition of nanoparticles.2 Fereidoon et al., have used carbon nanotubes (CNTs) and nano-alumina to improve morphology as well as thermal and water resistance of the poly(urea-formaldehyde) (PUF) shell walls of microcapsules filled with DCPD.13 Sarkar et al., used GO in the core epoxy and acetone solvent mixture to improve thermal properties and shelf life of epoxy-poly(urea-formaldehyde) microcapsules.3, 14 Fan et. al., produced hexadecane loaded microcapsules with GO/ PUF hybrid shells with poly (ethylene-alt-maleic anhydride) (EMA) emulsifier agent. They reported that good emulsifying ability of GO sheets and improved thermal and barrier properties of microcapsules produced by ultrasonication.15
Although there are many studies on GO- reinforced microcapsules,16-19 studies on GO- reinforced epoxy-loaded PUF microcapsules without emulsification agent for self-healing applications are limited. In this study, GO nanoparticles were added to the microencapsulation process without any pre-treatment and microcapsules were produced in one step in situ-emulsion polymerization method without emulsification agent. Then, the effect of GO on the chemical structure, morphology, and thermal behaviour of the produced microcapsules was investigated.
Experimental
Materials
Urea, formaldehyde (37% aqueous solution), ammonium chloride, resorcinol and graphite powder (ultra-thin grade) were analytical grade and purchased from Sigma-Aldrich Co, US. Epoxy (EP-100) resin was purchased from Hobbyart company, Turkey. All chemicals used without any purifications.
Experimental
Graphene oxide (GO) was produced according to modified Hummer’s method20 of our research group.21 Microcapsules were synthesized by in situ emulsion polymerization method without an emulsifier.9 Urea (0.04 mol), ammonium chloride (0.12 g) and resorcinol (0.12 g) dissolved in 40 ml deionized water (DI). Resorcinol as a crosslinking agent and ammonium chloride were added to improve the shell formation and strength of microcapsules.3, 22 Then a certain amount of GO added into 10 ml DI-water. GO suspension homogenized by 5 minutes sonication with ultrasonicator. The amounts of GO were determined according to to the total weight of shell monomers 0.25%, 0.5% and 1% wt/wt shell material. GO-suspension was added into microcapsule solution. pH of the solution fixed to 3.5 with 1 M HCl aqueous solution. Then 10 mL epoxy resin was added dropwise into vigorous mixing microcapsule solution. The emulsion solution stabilized for 15 minutes mixing at 800 rpm with a mechanical stirrer. 0.076 mol formaldehyde solution was added into the reaction solution. The temperature of the reaction solution was slowly increased to 60 °C with 1 °C/min rate. The reaction was conducted for 4 hours with mixing at 800 rpm. The reaction solution was cooled to ambient temperature then filtrated through vacuum filtration. The obtained microcapsules were purified with excess acetone and DI-water and dried at room temperature.
Characterization
The chemical structure, thermal properties and morphology of GO reinforced microcapsules and unreinforced microcapsules were characterized with Fourier transform infrared (FTIR) spectrometer (Thermo scientific Nicolet i550), thermal gravimetric analysis (Perkin-Elmer STA 6000) (TGA )and, scanning electron microscopy (SEM) (Carl Zeiss / Gemini 300), respectively. TGA analysis was done between 30 °C– 800 °C with 10 °C/min rate in N2 atmosphere.
Results and Discussion
Chemical Structure of GO- reinforced PUF-Epoxy Microcapsules
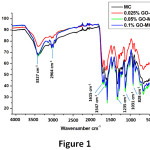
FT-IR spectrums of GO-reinforced and unreinforced PUF-epoxy microcapsules were shown in Figure 1. Characteristic peaks of poly(urea-formaldehyde) shell were seen at 3327cm-1 (N-H stretching), 1615cm-1(C=O bending of urea) and 1547cm-1(N-H bending of urea). Epoxy as core compound is detected at 1031 cm-1 (ether stretching of epoxy), 1235 cm-1(alkyl aryl ether stretching), 1500 cm-1 (C-O-C stretching of phenyl ring) and 828 cm-1, 914 cm-1(C-H and C=C bending of benzene,respectively), 2964 cm-1and 2926 cm-1( CH3 symmetric and asymmetric stretching of methyl groups, respectively).12 GO could not characterize due to peak overlapping21 and its very low concentration.
Figure 1: FT-IR spectrums of GO-reinforced and unreinforced PUF-epoxy microcapsules.
Thermal Behavior of GO- reinforced PUF-Epoxy Microcapsules
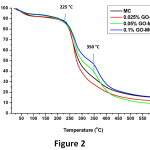
Thermal analysis results were shown in Figure 2. The first degradation temperature for all samples was 225 °C, so, this result shows that produced PUF-epoxy microcapsules are resistant up to 225 °C.9 Moreover, GO reinforced microcapsules show two-step degradation behaviour that proves the GO existence. This two-step degradation behaviour could not be seen in 0.25 GO-MC samples since the amount of GO is too low to effect the thermal behaviour. Moreover, 2nd step degradation begins at 350 °C that indicates degradation of the GO and epoxy in the of GO-reinforced microcapsules.9 1% GO-MC has more weight loss ratio at 2nd degradation step due to higher GO concentration.23
Figure 2: TGA thermograms of GO-reinforced and unreinforced PUF-epoxy microcapsules.
Morphology of GO- reinforced PUF-Epoxy Microcapsules
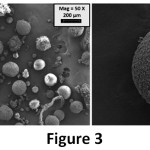
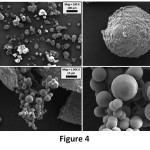
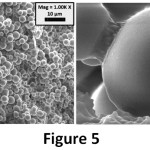
The reinforcing effect of GO on microcapsule was analysed in terms of size, morphology and shell thickness by using SEM analysis. Un-reinforced microcapsules (MC) were also shown in Figure 3. The surfaces of MC samples are rough and the shapes of microcapsules are straight. Yuan et. al., obtained similar microcapsules with a two-step polymerization method by using sodium dodecylbenzene sulfonate (SDBS) as emulsifying agent.24 On another hand, two different microcapsules exist in the 0.25 GO-MC sample (Figure 4); one of them is rougher with bigger size, the other one is smooth with small size. Moreover, 0.5 GO-MC samples (Figure 5) have just one type of microcapsules which is smooth with small size. This situation shows that GO addition significantly affects the microcapsule formation process.
GO acts as a surfactant in the microcapsule production process due to its high surface energy. GO addition to the microencapsulation process decreases the interfacial tension between epoxy and aqueous shell reaction solution and results in smaller epoxy droplets in the emulsion solution.15 Therefore, it alters the microcapsules’ shell morphology from rough to smooth and decreases the size of microcapsules and narrows the size distribution. While the average size of MC is approximately 140 µm, in 0.25 GO-MC and 0.5 GO-MC samples, size decreases to 3- 3.5 µm. In the literature, the microcapsules in that size (1-5 µm) produced with very high mixing rates (1500- 2000 rpm) or ultrasonic homogenization and also by the help of emulsifiers.10, 25 Sarkar et al., have produced microcapsules with urea–formaldehyde (UF) and GO–epoxy resin by using EMA as an emulsifier. They investigated the microcapsule production parameters such as stirring rate, core-shell content etc.3 However, in our study, we showed that the emulsification effect of GO addition to the microencapsulation process and the small-sized microcapsules can be obtained by a very low amount of GO addition without the need of high rate agitation or surfactant.
Figure 3: SEM images of the MC sample.
Figure 4: SEM images of 0.25 GO-MC sample.
Figure 5: SEM images of 0.5 GO-MC sample.
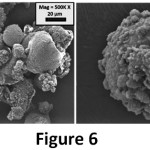
Figure 6: SEM images of 0.5 GO-MC sample.
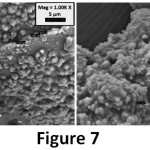
On the other hand, in the 0.1 GO-MC samples (Figure 6), the smooth and small microcapsules disappeared and very rough and layered microcapsules were seen. This situation shows us the surfactant effect of GO has an optimum concentration. When GO amount was increased, the morphology of microcapsules has changed and GO layers deposited onto the shell of the microcapsules. Also, the shell thickness of microcapsules increased from 1.47 to 1.52 by addition of 1% GO to the microcapsules (Figure 7). While the shell thickness of the microcapsules indicated existence on the shell structure, moreover, the size of microcapsules decreased from 140 µm (MC) to 35 µm (0.1 GO-MC). Fereidoonet. al. obtained similar results showing microcapsule size reduction with CNT addition in PUF-DCPD microcapsules.13 While GO–reinforced microcapsules were produced via grafting GO to the shell with expensive compounds (CTAB)17 in this study, GO-reinforced PUF/epoxy microcapsules were produced via an easy (one step) and cost-effective (without emulsifier) method.
Figure 7: Shell thicknesses of MC (1.47 µm) and 1% GO-MC (1.52 µm).
Conclusion
Graphene oxide reinforced epoxy loaded poly(urea-formaldehyde) microcapsules were produced by one step in-situ emulsion polymerization method without emulsifier agent. GO was incorporated into microcapsules without pre-treatment or ultra-speed agitation step. By using this easy and cost-effective method, application microcapsule-based self-healing property to various material can be facilitated. Analysis results revealed that obtained microcapsules are resistant up to 225 °C and also, second degradation step exists in GO reinforced microcapsules that indicate GO existence and thermal resistance of microcapsules. Addition of a certain amount of GO (0.5% wt/wt), results in acting of GO nanoparticles as a surfactant. Therefore, pretty small (3-4 µm) microcapsules were produced with low size distribution and smooth surface morphology. On the other hand, an increasing amount of GO to 1% results in higher shell thickness due to GO layers acting as a coating layer on the microcapsule shell. Moreover, 1% GO addition decreased the size of the microcapsules from ~140 µm to ~34 µm. Produced GO-reinforced epoxy loaded microcapsules have the potential to be used in self-healing composites and anticorrosion coatings.
References
- Yang T., Zhang J., Mouritz A., Wang C., Healing of carbon fibre–epoxy composite T-joints using mendable polymer fibre stitching. Composites Part B: Engineering. (2013); 45: 1499-1507.
CrossRef
- D.Y. Zhu, M.Z. Rong, M.Q. Zhang, Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Progress in Polymer Science. (2015); 49: 175-220.
CrossRef
- Sarkar S., Kim B., Synthesis of graphene oxide–epoxy resin encapsulated urea–formaldehyde microcapsule by in situ polymerization process. Polymer Composites. (2018); 39: 636-644.
CrossRef
- Brown E.N., Sottos N.R., White S.R., Fracture testing of a self-healing polymer composite. Experimental Mechanics. (2002); 42: 372-379.
CrossRef
- Wang R., Hu H., Liu W., Guo Q., Preparation and characterization of self-healing polymeric materials with microencapsulated epoxy and imidazoline derivatives curing agent. Polymers and Polymer Composites. (2011); 19: 279-288.
CrossRef
- Caruso M.M., Blaiszik B.J., White S.R., Sottos N.R., Moore J.S., Full recovery of fracture toughness using a nontoxic solvent‐based self‐healing system. Advanced Functional Materials. (2008); 18: 1898-1904.
CrossRef
- Blaiszik B., Caruso M., McIlroy D., Moore J., White S., Sottos N., Microcapsules filled with reactive solutions for self-healing materials. Polymer. (2009); 50: 990-997.
CrossRef
- Yuan Y.C., Rong M.Z., Zhang M.Q., Chen J., Yang G.C., Li X.M., Self-healing polymeric materials using epoxy/mercaptan as the healant. Macromolecules. 41, 5197-5202 (2008).
CrossRef
- Liao L., Zhang W., Xin Y., Wang H., Zhao Y., Li W., Preparation and characterization of a microcapsule containing epoxy resin and its self-healing performance of anticorrosion covering material. Chinese Science Bulletin. (2011); 56: 439-443.
CrossRef
- Safaei F., Khorasani S.N., Rahnama H., Neisiany R.E., Koochaki M.S., Single microcapsules containing epoxy healing agent used for development in the fabrication of cost-efficient self-healing epoxy coating. Progress in Organic Coatings. (2018); 114: 40-46.
CrossRef
- Zhao Y., Zhang W., Liao L.-p., Wang S.-j., Li W.-j., Self-healing coatings containing microcapsule. Applied Surface Science. (2012); 258: 1915-1918.
CrossRef
- Ting Z., Min Z., Xiao‐Mei T., Feng C., Jian‐Hui Q., Optimal preparation and characterization of poly (urea–formaldehyde) microcapsules. Journal of applied polymer science. (2010); 115: 2162-2169.
CrossRef
- Fereidoon A., Ahangari M.G., Jahanshahi M., Effect of nanoparticles on the morphology and thermal properties of self-healing poly(urea-formaldehyde) microcapsules. Journal of Polymer Research. (2013); 20: 151.
CrossRef
- Sarkar S., Kim B., Analysis of graphene-encapsulated polymer microcapsules with superior thermal and storage stability behaviour. Polymer Degradation and Stability. (2017); 138: 72-81.
CrossRef
- Fan C., Wei T., Hu C., Zhou X., Preparation of GO/PUF hybrid shell microcapsules using GO sheets as the particulate emulsifier. Micro & Nano Letters. (2016); 11: 207-211.
CrossRef
- Zhang L., Zhang Y., Xu H., Wang H., Du Q., Polymer/graphene oxide composite microcapsules with greatly improved barrier properties. RSC Advances. (2016); 6: 7618-7625.
CrossRef
- Qiao Z., Mao J., Multifunctional poly (melamine-urea-formaldehyde)/graphene microcapsules with low infrared emissivity and high thermal conductivity. Materials Science and Engineering: B. (2017); 226: 86-93.
CrossRef
- Wu B., Zheng G., Chen X., Effect of graphene on the thermophysical properties of melamine-urea-formaldehyde/N-hexadecane microcapsules. RSC Advances. (2015); 5: 74024-74031.
CrossRef
- Liu Z., Chen Z., Yu F., Microencapsulated phase change material modified by graphene oxide with different degrees of oxidation for solar energy storage. Solar Energy Materials and Solar Cells. (2018); 174: 453-459.
CrossRef
- Chen J., Yao B., Li C., Shi G., An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon. (2013); 64: 225-229.
CrossRef
- Tas M., Altin Y., Bedeloglu A., Graphene and graphene oxide-coated polyamide monofilament yarns for fibre-shaped flexible electrodes. The Journal of The Textile Institute. (2018); 1-7.
- Fan C., Tang J., Zhou X., Role of ammonium chloride in preparing poly (urea‐formaldehyde) microcapsules using the one‐step method. Journal of Applied Polymer Science. (2013); 129: 2848-2856.
CrossRef
- Park S., An J., Potts J.R., Velamakanni A., Murali S., Ruoff R.S., Hydrazine-reduction of graphite-and graphene oxide. Carbon. (2011); 49: 3019-3023.
CrossRef
- Yuan L., Liang G., Xie J., Li L., Guo J., Preparation and characterization of poly (urea-formaldehyde) microcapsules filled with epoxy resins. polymer. (2006); 47: 5338-5349.
- Cosco S., Ambrogi V., Musto P., Carfagna C., Properties of poly (urea‐formaldehyde) microcapsules containing an epoxy resin. Journal of applied polymer science. (2007);105: 1400-1411.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Ayse Celik Bedeloglu1*
, Ayse Celik Bedeloglu1*
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal