Carlos Dario Lopez Ramirez, Dairo E. Chaverra, Oscar Jaime Restrepo Baena*
Minerals Institute, CIMEX, School of Mines, Universidad Nacional de Colombia
Corresponding Author E-mail: ojrestre@unal.edu.co
DOI : http://dx.doi.org/10.13005/msri/160106
Article Publishing History
Article Received on : 21-Mar-2019
Article Accepted on : 22-Apr-2019
Article Published : 23 Apr 2019
Plagiarism Check: Yes
Reviewed by: Kalai S
Second Review by: Anuj Saini
Final Approval by: Xuan Dung Mai
Article Metrics
ABSTRACT:
Cyanide is one of the most used reagents in the precious metal extraction process; as well as the most efficient from the point of view of the dissolution process, but it is also a toxic product that requires a lot of care in handling. Likewise, the residual solutions of the process must be followed because they can be a risk of contamination of water, animals and human health. In the artisanal processes of obtaining gold and silver, neutralization of the residual solutions is used to passivate the present cyanide. During this process ammonium cyanate is formed which decomposes rapidly in the presence of air and sunlight in carbon dioxide and ammonia gas, contributing to the greenhouse effect. In this work, the use of the ammonium cyanate obtained in the process of neutralization of the cyanide solutions as a reagent to obtain urea is proposed. Urea was obtained indirectly through the use of the reagent kit UREA/BUN-COLOR. The process is effective at pH ≤ 4.5 with a rapid increase in solution temperature and the addition of hydrogen peroxide. The urea crystals begin to form at 50°C. The cyanide/urea ratio obtained was 1/7.5.
KEYWORDS:
Artisanal Mining; Cyanide Leaching; Effluent Treatment; Urea
Copy the following to cite this article:
Ramirez C. D. L, Chaverra D. E, Baena O. J. R. Obtaining Urea from Effluents of Gold Cyanidation Process. Mat. Sci. Res. India; 16 (1).
|
Copy the following to cite this URL:
Ramirez C. D. L, Chaverra D. E, Baena O. J. R. Obtaining Urea from Effluents of Gold Cyanidation Process. Mat. Sci. Res. India; 16 (1). Available from: https://bit.ly/2IPU8Y7
|
Introduction
In the processes of extractive gold metallurgy, the use of cyanide leaching is perhaps the most widely used method worldwide; by the ease of operation, its low cost and high levels of precious metal recovery. However, neutralization, in most processing plants, is not done properly, and leftovers of the solution with free cyanide are dumped in concentrations harmful to the environment, in particular animals and humans. Cyanide can enter surface water through releases from industries using cyanides. Due to its high toxicity, the presence of cyanide in water is a matter of great concern(Jawale et al., 2017).
The pollution generated by the effluents from the leaching of gold ores is one of the main obstacles of social opposition presented by mining activity due to the impact of both surface and groundwater. During the cyanide decomposition process, a compound known as ammonium cyanate OCN(NH4), which is a raw material for the manufacture of urea, CO(NH2)2 is generated.
In the case of good neutralization, cyanide is no longer a toxic compound through oxidizing reagents, but as a result, there is the generation and emission of ammonia and carbon dioxide into the atmosphere, generating both a greenhouse effect (Gómez, 2012). This is evidenced by the strong effervescence of the reaction, and a penetrating smell of ammonia, which can irritate respiratory mucous membranes and eyes; however because they are greenhouse gases they are considered pollutants.
The processes that are currently applied for the neutralization of cyanide, seek oxidation of the same. Among the most used processes to oxidize cyanide is: treatments with ozone, potassium permanganate, chlorine, sodium hypochlorite, Caro acid, titanium dioxide, quicklime, hydrogen peroxide, aeration,biological process, among many more(Dash et al., 2009; Gaviria and Meza, 2006; Jawale et al., 2017; Kitis et al., 2005; Potivichayanon et al., 2017). However, in the industrial environment, the best technical procedures are those of alkaline chlorination (sodium hypochlorite), hydrogen peroxide and ozone. In all these procedures the cyanate ion is generated, which in the presence of UV radiation and being half accused is transformed into ammonium cyanate. The latter decomposes rapidly into ammonia and carbon dioxide according to the reaction:
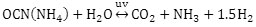
In the review of the literature, it was not evidenced by the existence of a process to obtain urea from the ammonium cyanate formed during the degradation of cyanidation solutions. However, urea is obtained at the industrial level through different processes like Stamicarbon and Thermo-Urea process, in which carbon dioxide CO2 and liquid ammonia are used. They are subjected to high pressure and high-temperature process. The reaction proceeds in two steps: in the first, the reagents form an intermediate compound called ammonium carbamate and in a second step the carbamate is dehydrated to form urea.
In this work, the process was developed to obtain urea from the transformation of ammonium cyanate from the treatment of effluents from the cyanidation process of gold minerals. For this purpose, the Wӧhler process was used, demonstrating that it is possible under controlled conditions to obtain said compound.
Materials and Methods
Cyanide solutions from discharges were used without previous neutralization. The samples, each 20 l, of effluents were taken from gold ore processing plants located at Segovia and Puerto Berrio in Antioquia, Colombia. They were transported under refrigeration conditions at 4°C with a pH of 11.35 and 11.5, for a period not exceeding 20 days.
The pH, free cyanide concentration in effluents and process temperature were evaluated. The formation of urea is given by the reaction:
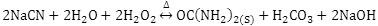
Triplicates test was prepared in the alkaline, neutral and acid ranges. Samples of 5 ml each was taken, at which the initial pH, initial temperature and [CN-] were measured by a colourimetric method with 0.1N silver nitrate. The calculation of hydrogen peroxide required for the neutralization of Cyanide (1) and the final pH was measured. In some cases, the solution was heated to T > 50 °C. Eleven tests were performed according to Table 1.
For the determination of the presence of urea and ammonium in each one of the tests the UREA/BUN-COLOR kit was used which allows a qualitative colourimetric evaluation of the presence of these compounds. This UREA/BUN-COLOR method is widely used in the veterinary clinical laboratory for the determination of urea and ammonium in urine, plasma and milk.
The method was applied as follows: hydrogen peroxide was first neutralized from a 5 ml sample of solution. Subsequently, a sub-sample of 10 μl was taken to which the different reagents were added to the urease in an amount of 1 ml each. If the result is blue Prussia indicates the presence of ammonium only. Finally, if a second sub-sample is added 1 ml of urease and the other reagents such as sodium hypochlorite, sodium salicylate and nitroprusside, resulting in a blue colouration of Prussia indicates the presence of urea.
Table 1: Experimental conditions for each test.
|
Test
|
pH
|
Temperature (°C)
|
[CN–]
|
H2O2added (ml)
|
Comments
|
|
1
|
5.00
|
22.8
|
1.5
|
52.5
|
Untilboiling
|
|
2
|
4.00
|
22.8
|
1.5
|
52.5
|
Untilboiling
|
|
3
|
11.35
|
22.8
|
1.5
|
–
|
No hydrogen peroxide or heating
|
|
4
|
4.40
|
22.8
|
1.5
|
52.5
|
Without heating
|
|
5
|
14.00
|
22.8
|
1.5
|
52.5
|
Without heating
|
|
6
|
6.60
|
25.0
|
0.8
|
3.00
|
Heatingtoevaporation
|
|
7
|
4.43
|
25.0
|
1.5
|
52.5
|
Heatingtoboiling
|
|
8
|
7.60
|
25.0
|
1.5
|
3.00
|
Heatingtoevaporation
|
|
9
|
4.00
|
23.0
|
1.7
|
59.5
|
Heatingtoevaporation
|
|
10
|
4.00
|
23.0
|
1.7
|
59.5
|
|
11
|
4.00
|
23.0
|
1.7
|
59.5
|
Results and Discussion
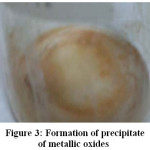
The main results are summarized in Table 2. The results for crystal formation, urea and ocher precipitate due to the presence of metal oxides are shown in Figures 1, 2 and 3.
Table 2: Results obtained in each test
|
Test
|
Comments
|
|
1
|
Formation of crystals. Formation of ammonium and urea
|
|
2
|
Formation of crystals. Formation of urea
|
|
3
|
AmmoniumFormation
|
|
4
|
No crystals, ammonium or urea
|
|
5
|
No crystals, ammonium or urea
|
|
6
|
Precipitateocher colour. No urea
|
|
7
|
No crystals, ammonium or urea
|
|
8
|
Precipitateocher colour. No urea
|
|
9
|
Formation of white crystals and ocher precipitate. Formation of urea.
|
|
10
|
|
11
|
Figure 1: Formation of crystals.
Figure 2: Positive result for urea formation.
Figure 3: Formation of a precipitate of metallic oxides.
From the results obtained experiments can establish the following:
• In the pH range 8-14, no ammonium is present or the formation of ammonium cyanate or urea is not possible.
• Ammonium and ammonia coexist in the pH 6.5 – 7.5 range but there is no ability to perform the hydrolysis of ammonium cyanate even by supplying heat.
• In the acid range, pH less than 4.5, the ammonium cyanate is formed immediately hydrogen peroxide is added. This has a very short duration, only 5 min, before decomposing into CO2 and NH3 which are emitted into the atmosphere.
• Formation of urea crystals alone is possible when the solution is heated within the first few seconds of the cyanide oxidation reaction with hydrogen peroxide. This allows hydrolysis of the ammonium cyanate as it is formed. The heating must be fast to avoid the generation and emission of CO2 and NH3, this because the reaction is endothermic at ambient temperature conditions.
• If the oxidation reaction is allowed to complete, approximately 2.5 h, it is no longer possible to obtain urea in the process. This is due to the spontaneous decomposition of the ammonium cyanate at room temperature.
• The formation of the first crystals of urea is around 50 ° C and accelerates as it gains heat, ΔH = -193.02 kcal.
• During the formation process of ammonium cyanate, the precipitation in the form of oxides and hydroxides of the metals dissolved in the solution is observed. Initially, they form an ocher colour film that floats in the solution and precipitates on the crystals of urea but without forming a mixture of visible.
• The rate of conversion of cyanide to urea in this process was 1: 7.5 taking into account that the addition of the oxidant was first performed and the heat was immediately supplied.
Conclusions
It is possible to obtain urea from the solutions of cyanide from the leaching of auroargentiferous minerals, taking advantage of the oxidation of cyanide. It should be heated immediately to boiling to accelerate the hydrolysis of the ammonium cyanate formed in the oxidation process as an intermediate step of short duration.
The pH of the neutralized solution should be ≤ 4.5 and the heating temperature should be higher than 50 °C for the formation of urea crystals.
The formation of ammonium cyanate is immediate and its decomposition is quite rapid; which is why it is difficult to determine that rate of transformation.
This process can be more economical if it takes advantage of the heat generated in the foundry furnaces.
The urea obtained is undoubtedly an important product extracted from a waste that currently pollutes the water and air. It is a different alternative to oil and living organisms. It could also be used in the recovery of soils degraded by gold mining, thus reducing the harmful effects of such economic activity.
It is convenient to analyze the quality of the urea obtained in particular, to verify traces of heavy metal elements, such as Hg, Pb, Cu, Cd, As, which are harmful to the environment.
Depending on the quality of the urea obtained, pilot experimentation could be achieved in agricultural use. Emphasizing that all urea used in Colombia is mainly imported from China and Russia.
Acknowledgements
The author wants to acknowledge Minerals Institute, CIMEX, School of Mines, Universidad Nacional de Colombia for all the support.
Funding Source
The Author declares no Funding Source.
References
- Dash R.R., Gaur A., Balomajumder C., 2009. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 163: 1–11. doi:10.1016/j.jhazmat.2008.06.051
CrossRef
- Gaviria A., Meza, L., 2006. Analysis of Alternatives for the Degradation of the Cyanide in Liquids and Solids Efluentes of the County of Segovia , Antioquia and in the Ore Dressing Mill of the Mineros Nacionales, County of Marmato, Caldas. Rev. Dyna 73: 31–44.
- Gómez P., 2012. Degradación de Cianuros mediante Oxidación Química en Efluentes Industriales. Universidad de Oviedo.
- Jawale R.H., Tandale A., Gogate P.R., 2017. Novel approaches based on ultrasound for the treatment of wastewater containing potassium ferrocyanide. Ultrason. Sonochem. 38: 402–409. doi:10.1016/j.ultsonch.2017.03.032
CrossRef
- Kitis M., Akcil A., Karakaya E., Yigit N.O., 2005. Destruction of cyanide by hydrogen peroxide in tailings slurries from low bearing sulphidic gold ores. Miner. Eng. 18: 353–362. doi:10.1016/j.mineng.2004.06.003
CrossRef
- Potivichayanon S., Supromin N., Toensakes R., 2017. Development of a mixed microbial culture for thiocyanate and metal cyanide degradation. 3 Biotech 7, 191. doi:10.1007/s13205-017-0814-6
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal