DC Conductivity and Transference Number in Pure and Potassium Thiocyanate-Doped Polyvinyl Alcohol Films
N. Nagaraj1, P. Mohan Babu2* and K. V. Ramesh Babu2
and K. V. Ramesh Babu2
1Department of Physiscs, S.K.R. Government Degree College, Gudur, Andrapradesh, INDIA.
2 Department of BS & H, QIS College of Engineering & Technology, Ongole, Andrapradesh, INDIA-523272
Corresponding Author Email: mohanbabu.qiscet@gmail.com
DOI : http://dx.doi.org/10.13005/msri/160207
Article Publishing History
Article Received on : 30-June-2019
Article Accepted on : 27-July-2019
Article Published : 29 Jul 2019
Plagiarism Check: Yes
Reviewed by: Amin Rabiei B
Second Review by: Raana Sarvari
Final Approval by: K. M. Garadkar
Article Metrics
ABSTRACT:
Poly (vinyl alcohol) (PVA) – based solid electrolyte films with potassium thiocyanate (KSCN) were prepared by solution-cast technique. The pure and KSCN- doped PVA films have been investigated for the charge transport mechanism in the polymer electrolyte system by using the DC conductivity (The composition dependence and temperature dependence in 300-385K range) and transference number measurements. The graphs related to conductivity – temperature shows that an increase in conductivity with respect to rise in the temperature. At room temperature, the conductivity of the (PVA+KSCN) electrolyte is 102 times greater than that of pure PVA. The transference number data exhibit that the charge transport in this polymer electrolyte system is predominantly due to ions. The ionic transference numbers (tion) lies in the range of 0.92 to 0.99 for the films of PVA with KSCN in the wt% ratios (90:10), (80:20) and (70:30).
KEYWORDS:
Composition dependence of DC conductivity; KSCN- doped polyvinyl alcohol films; Solution cast-technique; Transference numbers
Copy the following to cite this article:
Nagaraj N, Babu P. M, Babu K. V. R. DC Conductivity and Transference number in pure and potassium thiocyanate-doped polyvinyl alcohol films. Mat. Sci. Res. India;16 (2).
|
Copy the following to cite this URL:
Nagaraj N, Babu P. M, Babu K. V. R. DC Conductivity and Transference number in pure and potassium thiocyanate-doped polyvinyl alcohol films. Mat. Sci. Res. India;16 (2). Available from: http://bit.ly/2JYqyzT
|
Introduction
Solid polymer electrolytes i.e. polymer salt complexes are having great significance in various electrochemical devices such as fuel cells, high-energy batteries,display devices and gas sensors etc.1-4 Because of their wide range of composition, having good quality of interfacial contact between the electrode-electrolyte and ease of preparation polymers are more preferable over crystalline materials. In particular, polyvinyl alcohol (PVA) is an unique polymer which can deliquesce high concentrations of a wide variety of salts to form polymeric electrolytes. In recent times appreciable work has been reported on the charge carrier transport in doped polyvinyl alcohol films.5
In the work, we have fabricated solid polymer electrolyte films of PVA and (PVA+KSCN). To improve the ionic nature of the polymer we have chosen this electrolyte system by adding KSCN as a dopant because of its ability to provide additional charge carriers and its reluctance to chemically react with PVA.
The experimental techniques such as composition dependent conductivity in the wt% ratio (90:10), (80:20) and (70:30), temperature dependent conductivity in the temperature range 300-385K and Wagner’s polarization method were utilized to determine the conductivity and transference numbers of these polymer electrolyte films.
Experimental
The technique employed for the preparation of thin polymer films in the present study is the solution-cast technique. The polymer PVA is the host and potassium thiocyanate is dissolved in triple distilled water which is taken as solvent in adequate reciprocal composition. The two solutions are then mixed and stirred at room temperature for 6-8 hours to obtain homogeneous mixture. The mixture is cast on to polypropylene dishes and allowed to evaporate in hot air oven to obtain desired polymeric complex.The films were then dried in a vacuum of 10-3 torr to eliminate all the traces of the solvent.The films were then dried in a vacuum of 10-3torr to eliminate all the traces of the solvent.6-8 The thickness of the polymeric films (˜150µm) was measured by adapting capacitance method using an LCR bridge (TF 1313A).
Using an electrometer amplifier (EA 815 Supplied by ECIL, Hyderabad), the conductivity was measured in the temperature range 300-385K. By Wagner’s polarization technique, the ionic and electronic transport number (tion,t ele) were evaluated. A freshly prepared film of (PVA+KSCN) was polarized in a configuration of K/(PVA+KSCN)/C under a bias (A step potential of 1.5 V). The resulting current was recorded as a function of time with the help of Keithley electrometer (Model 604).
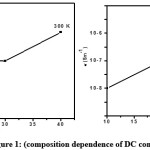
Figure 1: Composition dependence of DC conductivity of (PVA+KSCN) polymer films at various temperatures.
Table for figure-1: (Composition dependence of DC conductivity)
|
S.No.
|
Polymer Electrolyte System
|
Conductivity (Sm-1)
|
|
300K
|
330K
|
359K
|
385K
|
|
1
|
PVA
|
3.16 x 10-9
|
4.19 x 10-9
|
1.18 x 10-8
|
2.39 x 10-8
|
|
2
|
(PVA + KSCN) (90:10)
|
4.18 x 10-8
|
5.40 x 10-8
|
7.14 x 10-8
|
1.61 x 10-7
|
|
3
|
(PVA + KSCN) (80:20)
|
5.68 x 10-8
|
6.24 x 10-8
|
1.36 x 10-7
|
1.89 x 10-7
|
|
4
|
(PVA + KSCN) (70:30)
|
3.66 x 10-7
|
4.78 x 10-7
|
1.02 x 10-6
|
1.74 x 10-6
|
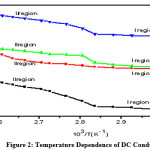
Figure 2: Temperature dependence of DC conductivity of (a) Undoped PVA (b) (PVA+KSCN) (90:10); (c) (PVA+KSCN) (80:20); (d) (PVA+KSCN) (70:30) films.
Table for figure-2: (Temperature Dependence of DC Conductivity)
|
S.NO
|
Undoped PVA
|
PVA +KSCN (90:10)
|
PVA +KSCN (80:20)
|
PVA +KSCN (70:30)
|
|
103/T
|
log (σT)
|
103/T
|
log (σT)
|
103/T
|
log (σT)
|
103/T
|
log (σT)
|
|
1
|
2.505
|
-5.02
|
2.505
|
-4.22
|
2.5.5
|
-4.14
|
2.505
|
-3.18
|
|
2
|
2.54
|
-5.08
|
2.54
|
-4.26
|
2.54
|
-4.18
|
2.54
|
-3.22
|
|
3
|
2.57
|
-5.1
|
2.59
|
-4.34
|
2.57
|
-4.2
|
2.57
|
-3.26
|
|
4
|
2.61
|
-5.16
|
2.61
|
-4.38
|
2.61
|
-4.22
|
2.61
|
-3.28
|
|
5
|
2.635
|
-5.22
|
2.635
|
-4.48
|
2.635
|
-4.24
|
2.635
|
-3.32
|
|
6
|
2.665
|
-5.28
|
2.665
|
-4.54
|
2.665
|
-4.28
|
2.665
|
-3.36
|
|
7
|
2.705
|
-5.34
|
2.705
|
-4.6
|
2.705
|
-4.32
|
2.705
|
-3.42
|
|
8
|
2.725
|
-5.4
|
2.725
|
-4.62
|
2.725
|
-4.36
|
2.725
|
-3.46
|
|
9
|
2.765
|
-5.54
|
2.765
|
-4.64
|
2.765
|
-4.38
|
2.765
|
-3.52
|
|
10
|
2.805
|
-5.68
|
2.805
|
-4.68
|
2.805
|
-4.4
|
2.805
|
-3.64
|
|
11
|
2.835
|
-5.82
|
2.835
|
-4.72
|
2.835
|
-4.6
|
2.835
|
-3.8
|
|
12
|
2.88
|
-5.84
|
2.88
|
-4.74
|
2.88
|
-4.66
|
2.88
|
-3.82
|
|
13
|
2.925
|
-5.86
|
2.925
|
-4.76
|
2.925
|
-4.7
|
2.925
|
-3.84
|
|
14
|
2.975
|
-5.9
|
2.975
|
-4.78
|
2.975
|
-4.72
|
2.995
|
-3.86
|
|
15
|
3.015
|
-5.92
|
3.105
|
-4.8
|
3.015
|
-4.74
|
3.015
|
-3.88
|
|
16
|
3.065
|
-5.94
|
3.065
|
-4.82
|
3.065
|
-4.76
|
3.065
|
-3.9
|
|
17
|
3.11
|
-5.96
|
3.11
|
-4.84
|
3.11
|
-4.78
|
3.11
|
-3.92
|
|
18
|
3.165
|
-5.98
|
3.165
|
-4.86
|
3.165
|
-4.78
|
3.165
|
-3.94
|
|
19
|
3.215
|
-6.02
|
3.215
|
-4.84
|
3.215
|
-4.8
|
3.215
|
-3.96
|
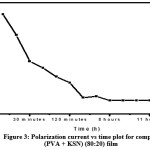
Figure 3: Polarization current vs time plot for complexed (PVA+KSCN) (80:20) film.
Table for figure-3 (Polarization current Vs. Time plot for complexed film (PVA(80) + KSN (20))
|
S.No.
|
Time ( h)
|
Polarization current (I)
|
|
1
|
0
|
6.2
|
|
2
|
0.1
|
4.8
|
|
3
|
0.5
|
3.04
|
|
4
|
1.0
|
2.6
|
|
5
|
1.5
|
2
|
|
6
|
2.00
|
1.6
|
|
7
|
2.8
|
0.6
|
|
8
|
5.8
|
0.68
|
|
9
|
8
|
0.4
|
|
10
|
9
|
0.4
|
|
11
|
10
|
0.4
|
|
12
|
11
|
0.4
|
|
13
|
12
|
0.4
|
|
14
|
13
|
0.4
|
|
15
|
14
|
0.4
|
Table 1: DC electrical conductivity of (PVA+KSCN) polymer electrolyte system at various Temperatures.
|
S.No.
|
Polymer electrolyte system
|
Conductivity (Sm-1)
|
|
300K
|
330K
|
359K
|
385K
|
|
1
|
PVA
|
3.16 x 10-9
|
4.19 x 10-9
|
1.18 x 10-8
|
2.39 x 10-8
|
|
2
|
(PVA + KSCN) (90:10)
|
4.18 x 10-8
|
5.40 x 10-8
|
7.14 x 10-8
|
1.61 x 10-7
|
|
3
|
(PVA + KSCN) (80:20)
|
5.68 x 10-8
|
6.24 x 10-8
|
1.36 x 10-7
|
1.89 x 10-7
|
|
4
|
(PVA + KSCN) (70:30)
|
3.66 x 10-7
|
4.78 x 10-7
|
1.02 x 10-6
|
1.74 x 10-6
|
Results and Discussion
Fig. 1 shows the composition dependence of electrical conductivity of PVA and (PVA+KSCN) polymer electrolytes at different temperatures. The conductivity values of PVA and (PVA+KSCN) electrolyte systems are given in table 1 for different temperatures. The following features were observed from the figure and the table.9-10
a) At room temperature the conductivity of pure PVA is about 10-9 Sm-1. And its value increases on complexing it with KSCN. The increase in conductivity is tenfold for a composition of 80:20 while it increases to 100 fold for a composition of 70:30.
b) The increase in conductivity with increase of KSCN is hold responsible for the decrease in the degree of crystallinity and increase in the degree of amorphosity.
The fig.2 shows the conductivity vs temperature graphs of pure PVA and different compositions of (PVA+KSCN) polymer electrolytes in the temperature range 300-385K. From the conductivity temperature plots, the following features are noticed.
a) It is found that, the conductivity increases with increase of temperature in pure PVA and in all the compositions of (PVA+KSCN) polymer electrolyte.
b) The conductivity-temperature graphs obey an Arrhenius kind of featuresall the way through, having two regions with different kind of activation energies, below and above (regions I &II) the glass transition temperature (Tg) of the polymer. It is observed that in region-I the conductivity increases slowly with temperature up to 720; form then onwards a sudden increase in conductivity was appeared. Similarly, in region- II also the conductivity increases with temperature. The same kind of behavior was observed for all compositions of the (PVA+KSCN) polymer electrolyte system.
Having two regions and the swift increase in the conductivity at Tg may because of the transition from semicrystalline phase to an amorphous phase. The increase of conductivity with temperature below Tg (region-I) is interpreted as a hopping mechanism between coordinating sites, local structural relaxations and segmental motions of the polymer. The amorphous nature in region-II favors the inter-chain and intra-chain ion movements and congruously the conductivity becomes high in the polymer electrolyte.
The following relation can be fitted for variation of electrical conductivity (σ) as a function of temperature (T) in the entire temperature range./11/.
(σT) = (σT)0exp(-Ea/KT)
Where (σT)o= The pre-exponential factor
Ea = The activation energy
K =The Boltzmann constant.
The values of calculated pre-exponential factor (σT)0 and activation energies (Ea) for (PVA+KSCN) complexes are summarized in Table 2.
Table 2: The pre-exponential factors (σT)0 and Activation energies (Ea) of (PVA+KSCN) polymer electrolyte system.
|
Polymer electrolyte system
|
Region – I
|
Region – II
|
|
Ea (eV)
|
(σT)0 (Sm-1 K)
|
Ea (eV)
|
(σT)0 (Sm-1 K)
|
|
PVA
|
0.18
|
9.44 x 10-4
|
0.25
|
1.70 x 10-3
|
|
(PVA + KSCN) (90:10)
|
0.11
|
9.89 x 10-4
|
0.12
|
2.32 x 10-3
|
|
(PVA + KSCN) (80:20)
|
0.13
|
2.47 x 10-3
|
0.19
|
2.13 x 10-2
|
|
(PVA + KSCN) (70:30)
|
0.15
|
3.62 x 10-2
|
0.2
|
2.78 x 10-1
|
By using Wagner’s polarization method, the transference numbers corresponding to ionic (tion) and electronic (tele) transport have been evaluated in (PVA+KSCN) electrolyte system. The fig 3 shows polarization current – time plot for complexed (PVA+KSCN) (80:20) film.Using the below equations,the transference numbers (tionand tele) are calculated.
tion = (Ii – If) / Ii
tele = 1 – tion
Where Ii=The initial current
If= The final residual current.
The resulting data was given in Table 3. From the data it was observed that, for all the compositions of the (PVA+KSCN) electrolyte system, the value of the ionic transference numbers (tion) are lies in the range 0.92 to 0.99. Hence, it will conclude that the charge transport in these polymer electrolyte films is predominantly due to ions; only a negligible contribution comes from the electrons.12-13
Table 3: Transference number data of (PVA+KSCN) polymer electrolyte System.
|
Polymer electrolyte system
|
Transference Number
|
|
tion
|
tele
|
|
(PVA + KSCN) (90:10)
|
0.92
|
0.08
|
|
(PVA + KSCN) (80:20)
|
0.94
|
0.06
|
|
(PVA + KSCN) (70:30)
|
0.99
|
0.01
|
Conclusion
The experimental analysis strongly reveals that KSCN is effectively doped with PVA to intensify its electrical conductivity. The conductivity of KSCN doped PVA electrolyte film is about 102 times greater than that of pure PVA. This is due to the increase in crystallanity in the PVA polymer by the addition of KSCN. Regarding the transference number, from the data on ionic transport numbers in these polymeric electrolyte films indicates that the charge transport is predominant due to ions.
Acknowledgements
The authors thank Prof. A.K. Sharma, Department of Physics, S.V.University, A.P. India. For his encouragement. The authors extend their thanks to Dr. Md. Hussain Basha (Senior Research Fellow), V.S University, (P.G Centre, Kavli), A.P. for his help on providing software for graphs.
Funding Source
One of the authors N. Nagaraj meet the funding by own.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article
References
- P. Balaji Bargav, V. Madhu Mohan, A.K. Sharma and V.V.R.N. Rao Ionics, 13(2007)441.
CrossRef
- G.K. Prajapayti, R.Roshan and P.N. Gupta Journal of Physics and Chemistry of Solids, 71(2010)1717.
CrossRef
- Arvind Awadhia and S.L. Agarwal. Solid State Ionic, 178(2007)951.
CrossRef
- CH.V.Subba Reddy, A.K.Sharma and V.V.R.N.Rao Journal of Power Sources, 111(2002)357.
CrossRef
- M.Hema, S.Selvasekarapandian and A.Sakuntala Condensed Physics, 403(2008)2740.
CrossRef
- V.M.Mohan, P.B.Bargav, V.Raja, A.K.Sharma and V.V.R.N.Rao Soft Materials, 5(2007)1.
CrossRef
- P.BalajiBargav, V.Madhu Mohan, A.K.Sharma and V.V.R.N.Rao Current Aplied Physics, 9(2009)165.
CrossRef
- M.Rani, Y.Pavani, K.Kiran Kumar, S.Bhavani,A.K.Sharma and V.V.R.N.Rao Materials Chemistry and Physics, 130(2011)442.
CrossRef
- CH.V.Subba Reddy, A.K.Sharma and V.V.R.N.Rao Journal of Material Science Letters, 21(2002)105.
CrossRef
- P. Balaji Bargav, V. Madhu Mohan, A.K. Sharma and V.V.R.N. Rao. International Journal of Polymeric Materials and Polymeric Biomaterials,(2006)579.
- C. Uma Devi, A.K. Sharma and V.V.R.N. Rao, Materials Letters, 56(2002)167.
CrossRef
- S. Ramesh and A.K. Arof Materials Science and Engineering, 85(2001)11.
CrossRef
- A.A.Ahamad, N.S.Ahamed and R.Othman Ionics, 156(2003)171.
CrossRef
- P. Balaji Bargav, B.A. Sarada, A.K. Sharma and V.V.R.N. Rao Journal of Macro Molecular Science (2009)131.
CrossRef
- K. Kumar, M.Ravi, Y.Pavani and S.Bhavani Journal of Non-Crystalline Solids, 358(2012)3205.
- M.B. Armand, Ann. Rev. Sci., 16(1986)245
CrossRef
- M.A.Ratner and Shriver, Chem. Rev. 88(1988)109
CrossRef
- J. Ulanski, P.Polanoiwski, A.Traiez, M.hofman, E.Dormann and E.Laukhina, Synth Met., 94(1988)23.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 and K. V. Ramesh Babu2
and K. V. Ramesh Babu2 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal