Evaluation of the Reactivity of Artificial Mixtures of Portland Cement Clinker Obtained by Flame Spray Pyrolysis
Andrés Felipe Pinto, Jorge Iván Tobón, Natalia Betancur-Granados and Oscar Jaime Restrepo-Baena*
Grupo del Cemento y Materiales de Construcción, Departamento de Materiales y Minerales, Universidad Nacional de Colombia Sede Medellín, Calle 59A No 63 – 20, Medellín 050036, Colombia.
Corresponding Author E-mail: ojrestre@unal.edu.co
DOI : http://dx.doi.org/10.13005/msri/160204
Article Publishing History
Article Received on : 30-Mar-2019
Article Accepted on : 25-May-2019
Article Published : 25 May 2019
Plagiarism Check: Yes
Reviewed by: Behzad Fotovvati
Second Review by: Haftom Gebrekiros
Final Approval by: Dr. Amelia Almeida
Article Metrics
ABSTRACT:
The Portland cement clinker consists of 95% calcium oxide, silicon, aluminium and iron and 5% impurities of magnesium, sodium, potassium, titanium, sulfur, phosphorus and manganese. From the combination of two or more of the main oxides, the constituents of the white clinker are formed, corresponding to alite(3CaO.SiO2 or C3S), belite (2CaO.SiO2 or C2S) and celite (Ca3Al2O6 or C3A), which give cement its characteristic properties. The fundamental properties of cement are its mechanical resistance, chemical resistance, the speed of reaction with water and the heat given off in hydration. In this work, the reactivity of an artificial mixture of white clinker, formed from alite, belite and celite prepared by flame spray pyrolysis was evaluated. The phases were characterized by X-ray diffraction, scanning electron microscopy and microcalorimetry, to evaluate their formation and reactivity. The characterization showed that during the synthesis of belite, a greater amount of the polymorph alpha was produced, with some impurities. On the other hand, the synthesis of celite allowed the production of the polymorph CII. The reactivity was evaluated by microcalorimetry.
KEYWORDS:
Clinker; Flame Spraypyrolysis; Nanoparticles; Oxidant Flame; Reactivity
Copy the following to cite this article:
Pinto A. F, Tobón J. I, Betancur-Granados N, Restrepo-Baena O. J. Evaluation of the reactivity of artificial mixtures of portland cement clinker obtained by flame spray pyrolysis. Mat. Sci. Res. India; 16 (2).
|
Copy the following to cite this URL:
Pinto A. F, Tobón J. I, Betancur-Granados N, Restrepo-Baena O. J. Evaluation of the reactivity of artificial mixtures of portland cement clinker obtained by flame spray pyrolysis. Mat. Sci. Res. India; 16 (2). Available from: https://bit.ly/2JD5CQ8
|
Introduction
Portland cement is a type of material obtained from the rotary kiln cooking of a mixture of limestone and clay, which is brought to sinter to form an intermediate product called clinker, which is mixed with plaster, giving rise to Portland cement.1
The clinker consists of 95% of calcium oxides, silicon, aluminium and iron and 5% of impurities of magnesium, sodium, potassium, titanium, sulfur, phosphorus and manganese. From the combination of two or more of the main oxides, the main constituents of the clinker are formed corresponding to alite (3CaO.SiO2 or C3S), belite (2CaO.SiO2 or C2S) and celite (Ca3Al2O6 or C3A), which give cement its characteristic properties.1 A typical mineralogical composition of clinker is formed by 70% alite, 22% belite, 8% celite.2
The traditional process for the synthesis of the clinker phases is by solid-state reaction or partial melting process3 which has an advantage the simplicity of the process and the disadvantages as high power consumption, high emissions of CO2 and slow diffusion processes that lead to the formation of inhomogeneous phase.4 Taking into account the above, according to N. Betancur-Granados,5 In recent years, non-conventional methods have been sought for the production of nanometric cementitious materials, with a high production ratio, which can provide greater reactivity in each of the clinker phases. Therefore, taking into account the high volumes required in the production of cement, it is necessary to look for alternative methods of synthesis of the Portland cement clinker phases, to processes that can be scaled industrially. In this sense, spray technologies have a great potential for use since they are continuous processes, which can be easily taken to industrial production, allowing to go from production of g/h to kg/h without changes in the characteristics of the particles.6-7
In this work, the reactivity of artificial clinker mixtures, formed from alite, belite and celite prepared by flame spray pyrolysis with liquid feed (FSP for its acronym in English) was evaluated. The formulation of the artificial mixture was chosen with respect to the mineralogical composition of a commercial white cement, in order to evaluate the reactivity. The products were characterized by X-ray diffraction, to determine the formation of the phases and the reactivity of the mixture was evaluated by microcalorimetry.
Experimentation
Materials
In this work, calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, 98%) from Baker, nonahydrate aluminium nitrate [Al(NO3)3·9H2O, 98.5%] from Merk, tetraethyl orthosilicate [SiC8H20O4, 99%] of Sigma Aldrich and ethanol [CH3CH2OH, 96%] of ProtoKimica was used.
Flame spray pyrolysis method
The clinker, belite (2Ca.SiO2 or C2S) and celite (Ca3Al2O6 or C3A) phases were synthesized through flame spray pyrolysis using metalorganic precursors and inorganic salts in stoichiometric amounts. On the other hand, the triclinic alite phase was acquired commercially in the company CTL company.
The starting solution to obtain 7 g of belite (2CaO.SiO2 or C2S) was prepared by dissolving 8.55 g (0.041 mole) of tetraethyl orthosilicate (SiC8H20O4) and 19.19 g (0.081 mole) of calcium nitrate (Ca(NO3)2·4H2O) in 355 mL of ethanol, with a ceramic load of 2.5%, the percentage of which corresponds to the mass ratio of the product (belite) and the solvent (ethanol). The solution was fed with a flow of 30 mL/min and a dispersion pressure of 200 kPa, to form a spray, by means of a compression nozzle directed towards acetylene/oxygen oxidant flame. The reaction took place in 12.5 minutes and the resulting powders were collected in an electrostatic precipitator with a voltage of 15 kV.
Similarly, to obtain 5 g of celite (Ca3Al2O6 or C3A), a solution was prepared with 13.11 g (0.056 mol) of calcium nitrate (Ca(NO3)2·4H2O) and 13.88 g (0.037 mol) of aluminum nitrate (Al (NO3)3·9H2O) in 127 mL of ethanol, with a ceramic load of 5%; the solution was fed with a flow of 50 mL/min and a dispersion pressure of 200 kPa, with an approximate reaction time of 4 minutes.
Reactivity of the artificial mixture
With the samples of alite, belite and celite a mixture of 1 g of clinker were prepared to take as a reference a typical composition for white Portland cement, with 70% alite (0.7074 g), 21% belite (0.2100 g) and 9% celite (0.0906 g). To measure the reactivity of the mixture was used a TAM Air microcalorimeter, which was introduced in 20 mL glass ampoules to make reactivity measurements, using air as a standard. The equipment was left in thermal stabilization for 24 hours. Water was then injected into the sample with a water / solid ratio of 0.87, using an admix device and mixed for two minutes. The heat released and the heat flow was measured for 8 continuous days.
Characterization
The mineralogical analysis was carried out by means of X-ray diffraction (XRD) in an XPert PANalytical Empyrean Series II-Alpha 1 diffractometer, 2012 model using Cu-kα radiation. The scans were continuous from 10° to 70° (2θ) with increments of 0.02 and 50 s per step. The quantification of the mineralogical phases was carried out using the Rietveld method of least squares, using the GSAS free software.
The morphologies were evaluated by scanning electron microscopy (SEM) in an EVO Model MA10, Carl Zeiss. The powders were dispersed in acetone for 10 minutes using an ultrasonic bath and deposited on a polished silicone support. The samples were coated with gold using a QT150R sputter, Quorum Technologies.
To measure the heat release and the reactivity of the sample, a TAM Air microcalorimeter from TA Instruments of an isothermal principle with an air thermostat at 25° C was used.
Results and discussion
In Fig. 1, the X-ray diffraction patterns of each of the synthesized phases are shown. The alite sample showed the presence of triclinic alite (code ICSD 98-016-2744), in addition, the formation of a monoclinic polymorph (code ICSD 98-008-1100) is shown. The formation of this polymorph can be due to the variation of the temperature of the reaction, since the alite can present different polymorphisms as the temperature increases, first a triclinic crystalline structure, then monoclinic and finally rhombohedral.8
The C2S sample showed mainly the formation of ofαL-C2S (code ICSD 98-008-2996). This sample obtained by FSP was then calcined at 800° C for 2 hours at a heating rate of 10° C / min, in order to achieve the complete formation of the crystalline phase. The peak located at 27.28 (2θ) belongs to low quartz (code 98-007-1393), which could be given as an impurity, during the calcination process, by a slight calcium deficiency in the formulation.
The celite sample showed the formation of cubic C3A II (code ICSD 98-015-1369), without the need for a subsequent thermal process, due to the high energy provided to the reaction, the volume of solution used, the feed flow of the solution and the similarity in the starting precursors, allow the reaction to develop in a short time. The peaks located at 34.1 and 53.8 (2θ) belong to Al2O3 (code ICSD 98-016-1061), which could be formed as a secondary product, by reacting with the oxygen excess of the oxidant flame used in the process.
Figure 1: X-ray diffraction patterns of the samples.
In Fig. 2, the SEM microscopy of the C2S phase synthesized by FSP is shown, where it is possible to observe an agglomerate of nanoparticles with spherical morphology and sizes around 100 nm, while a conventional belite is also rounded but has diameters around from 10 to 20 μm, so it is expected that the reactivity will increase appreciably. It is possible to identify a homogeneity in the distribution of the particle size, which is due to the fact that the flow used to feed the solution is moderate and therefore the contact between the precursor that comes into contact with the flame is adequate, otherwise, for a very high flow, there can be a high collision between particles affecting not only the structure of the flame but also an increase in the agglomeration.9
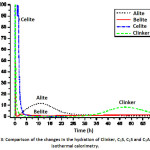
The reactivity of the clinker mixture and the individual phases was evaluated by isothermal calorimetry, which continuously measures the heat flow associated with physical processes and chemical reactions that occur during a phenomenon that requires exchanging heat with the external environment, as seen in Fig. 3. The calorimetric studies have revealed that the behaviour of the heat flow in the activation of these materials is represented by a curve of two peaks associating each of them with different reaction stages.10
Figure 2: SEM microscopy of the C2S.
Figure 3: Comparison of the changes in the hydration of Clinker, C3S, C2S and C3A during isothermal calorimetry.
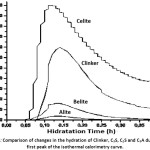
Figure 4 shows the first dissolution peak obtained by calorimetry for each of the phases, alite (C3S), belite (C2S), celite (C3A) and Clinker mixture respectively. This peak comprises two stages, one in which a rapid exothermic reaction occurs due to the initial hydrolysis and the release of calcium, silicon and aluminum ions and another in which there is a period of deceleration of the heat evolution due to the formation of both hydrated calcium silicate (CSH) and hydrated tricalcium aluminate (C3AH6) on the surface of the particles.11
When making a comparison between the four curves, the phase that presents a greater release of heat and reaches its maximum peak in a shorter time of hydration is the celite (C3A), because it is the phase that most affects the hydration kinetics of the clinker at early ages, apart from the alita (C3S).12 In addition, during this first period, the phase, once in contact with water, tends to undergo a solution, releasing calcium and aluminum ions to the surface of the particle of (C3A), which react with water molecules, forming hydrated tricalcium aluminate (C3AH6), in which reaction produces a high release of heat with respect to the other phases.
In the same way it can be observed that the peaks that follow the celite phase (C3A), in heat release are the mixture of clinker, belite (C2S) and finally the alite (C3S) respectively, reaching its maximum in the same hydration time at approximately 9 minutes, but the heat release value is different, since the release of heat is controlled by the rate of dissolution. Thus, it is shown that the belite (C2S) and celite (C3A) phases synthesized by flame spray pyrolysis method (FSP), having a nanometric particle size show a higher dissolution speed compared to the alite phase (C3S) which has a larger particle size because its synthesis method is conventional and therefore its dissolution speed is slower, showing a lower release of heat.
The first peak of the clinker mixture shows a high heat release, due to the dissolution speed of the belite (C2S) and celite (C3A) phases.
Figure 4: Comparison of changes in the hydration of Clinker, C3S, C2S and C3A during the first peak of the isothermal calorimetry curve.
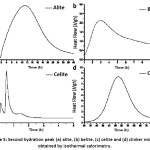
Fig. 5 (a) and (b) show the second peak of the calorimetric curve for alite (C3S) and belite (C2S), respectively, after the induction period has elapsed. During the first peak the dissolution of the phases occurred and the C-S-H was formed on the surface of the particles from the reaction between the calcium and silicate ions released on the surface with the water. When a saturation point is reached a first layer of (C-S-H) precipitates and occurs the deceleration stage of the heat evolution of the first peak and the induction stage where a slow dissolution of the particles continues and the concentration of dissolved units increases, which means that the liquid has diffused through all the material, they begin to generate pre-cores and possible cores for their subsequent growth and formation of the reaction products.10
As seen in Fig. 5 (a) and (b), the time required to reach the peak point of the second peak of the alite (C3S) is greater than for the belite (C2S), since the particle size has a direct relationship with its reaction speed. Considering that the C2S has a smaller size than C3S, the time of diffusion of the liquid throughout the particle is faster, causing that almost all of the Ca2+ and Si4+ ions have been released once the induction period has ended, therefore, the nucleation and growth of the CSH gel occurs more quickly.
Figure 5: Second hydration peak (a) alite, (b) belite, (c) celite and (d) clinker mixture obtained by isothermal calorimetry.
The calorimetric curve of the C3A celite Fig.5 (c) shows a shorter hydration time to reach its maximum peaks compared to the alite (C3S) and belite (C2S) phases. The heat released by the celite is very high which has an important effect on the initial hydration kinetics of the clinker.12 Once the phase comes in contact with the water, it starts a dissolution of the structure, releasing calcium and aluminium ions from the surface of the particles, which react with the water to form hydrated tricalcium aluminate (C3AH6), which generates a high heat release.
In Fig. 5 (d) it is observed that the second peak of the heat release curve for the clinker mixture requires a long period of induction to be formed in comparison with the individual phases, because when mixing and hydrating, the reaction rates of each component change with respect to those shown by the pure phases, giving place to a greater number of reactions and interaction of ions, which begin to compete with each other to reorganize and finally promote the formation of the C-S-H gel and hydrated tricalcium aluminate (C3AH6). One of the reasons why this second peak requires a longer formation time may be the precipitation of calcium aluminate hydrates on the surface of calcium silicates, inhibiting the formation of CSH, since there is no presence of gypsum, which helps to control the quick setting of celite.12
Conclusions
The X-ray diffraction results of the belite (C2S) and celite (C3A) phases show that the formation of the cementing phases is possible by flame spray pyrolysis method (FSP).
Once the study of the hydration of both the clinker mixture and its respective phases by isothermal microcalorimetry has been carried out, the main factor that intervenes in the hydration process is the dissolution speed of the particles, which depends on the particle size, because at smaller sizes of the order of nanometers the contact surface with the water increases and consequently the dissolution speed is greater. Thus, when making a comparison between the micrometric phase of alite (C3S) synthesized by the conventional solid-state reaction method and the nanite phase of belite (C2S) synthesized by flame spray pyrolysis method (FSP), belite showed a greater reactivity, since it was hydrated in less time with respect to the alite.
In relation to the clinker mixture, it was observed that its hydration requires a longer period of time compared to the phases that compose it, because there is a competition between the intrinsic chemical reactions of the process, due to a greater availability of ions of calcium in the medium and the formation of hydrated tricalcium aluminate (C3AH6).
Acknowledgement and Funding Source
Authors express their gratitude to the School of Mines of Universidad Nacional de Colombia and especially to the Research Group of the Cement and Building Materials for the support in carrying out the laboratory tests, as well as in the experimental support and financing of the tests carried out.
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- M. Ramayo Ramos, “Dimensionamiento de un horno rotatorio para la producción de Clínker de cemento Portland,” Univ. Cádiz, 2008.
- A. M. Castañon, S. García Granda, A. M. Guerrero, and F. Gómez-Fernández, “A research of the mineralogy phases of clinker in a Spanish cement using the method of Rietveld,” Dyna, 2012; vol. 79, no. 173: pp. 41–47.
- D. Stephan and P. Wilhelm, “Synthesis of pure cementitious phases by sol-gel process as precursor,” Zeitschrift fur Anorg. und Allg. Chemie, 2004; vol. 630, no. 10: pp. 1477–1483.
CrossRef
- J. C. Restrepo, A. Chavarriaga, O. J. Restrepo, and J. I. Tobon, “Synthesis of hydraulically active calcium silicates produced by combustion methods,” Mater. Res. Soc. Symp. Proc., 2015; vol. 1768: pp. 40–50.
CrossRef
- N. Betancur-Granados, J. I. Tobón, and O. J. Restrepo-Baena, “Alternative Production Processes of Calcium Silicate Phases of Portland Alternative Production Processes of Calcium Silicate Phases of Portland Cement : A Review,” 2018; Civ. Eng. Res. J., vol. 5, no. 3: pp. 1–6.
CrossRef
- X. Qin, Y. Ju, S. Bernhard, and N. Yao, “Europium-doped yttrium silicate nanophosphors prepared by flame synthesis,” Mater. Res. Bull., 2007; vol. 42, no. 8: pp. 1440–1449.
CrossRef
- A. J. Gröhn, S. E. Pratsinis, and K. Wegner, “Fluid-particle dynamics during combustion spray aerosol synthesis of ZrO2,” Chem. Eng. J., 2012; vol. 19: pp. 491–502.
CrossRef
- A. Wesselsky and O. M. Jensen, “Synthesis of pure Portland cement phases,” Cem. Concr. Res., 2009; vol. 39, no. 11: pp. 973–980.
CrossRef
- W. C. Jiménez, “Síntesis de Nanoestructuras de óxidos de hierro y Níquel por medio de llama difusa.,” PhD Propos., vol. 1, 2015.
- A. A. Hoyos Montilla, “Evolución de la reacción y estructura del sistema portlandita – ceniza volante de carbón activado alcalinamente Evolución de la reacción y estructura del sistema portlandita – ceniza volante de carbón activado alcalinamente,” 2018.
- D. Stephan, S. N. Dikoundou, and G. Raudaschl-Sieber, “Hydration characteristics and hydration products of tricalcium silicate doped with a combination of MgO, Al2O3 and Fe2O3,” Thermochim. Acta, 2008; vol. 472, no. 1–2: pp. 64–73.
CrossRef
- D. Marchon and R. J. Flatt, “Mechanisms of cement hydration,” Sci. Technol. Concr. Admixtures, 2015; vol. 41, no. 12: pp. 129–145.
CrossRef
- Zhang, B., Wei, J., Zeng, Z., Xu, W., & Yu, Q. (2018). Effects of Sulfur on the Solidification of Cadmium during Clinkerization. ACS Sustainable Chemistry & Engineering, 6(8): 10645-10653.
CrossRef
- Jiang, Y., Ling, T. C., Shi, C., & Pan, S. Y. (2018). Characteristics of steel slags and their use in cement and concrete—A review. Resources, Conservation and Recycling, 136: 187-197.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal