Gulsum AYDIN1, Kenan YILDIRIM2,3 and Ayse KALEMTAS4*
1Selcuk University, Faculty of Natural Sciences, Department of Biotechnology, Konya, Turkey
2Bursa Technical University Central Research Laboratory, Bursa, Turkey
3Bursa Technical University, Faculty of Engineering and Natural Sciences, Department of Fiber and Polymer Engineering, Bursa, Turkey
4Bursa Technical University, Faculty of Engineering and Natural Sciences, Department of Metallurgical and Materials Engineering, Bursa, Turkey
Corresponding Author E-mail: ayse.kalemtas@btu.edu.tr
DOI : http://dx.doi.org/10.13005/msri/160307
Article Publishing History
Article Received on : 21-11-2019
Article Accepted on : 16-12-2019
Article Published : 18 Dec 2019
Plagiarism Check: Yes
Reviewed by: Bharath V
Second Review by: Mahros Darsin Final Approval by: Alokmay Datta
Final Approval by: Alokmay Datta
Article Metrics
ABSTRACT:
In this study, a simple, innovative approach is applied to produce porous a-TCP-CeO2-Al2O3 composite beads via using bovine bone-derived hydroxyapatite, cerium oxide, and alumina ceramics. Bovine-bone derived hydroxyapatite was obtained via calcination of bones at 950°C for 3 hours. Hydroxyapatite is a thermally unstable biomaterial at high temperatures, and depending on its stoichiometry decomposes at 800-1200°C. Sodium alginate was successfully used as an in situ gelling templates for the production of the ceramic beads and starch, an environmentally friendly and economic pore-forming agent, is used to achieve interconnected, highly open porosity containing composite beads. Sintering of the ceramic−starch−alginate green composite beads at 1200°C for 1 hour resulted in the decomposition of the hydroxyapatite phase and formation of a-TCP. XRD analysis revealed that a-TCP-CeO2-Al2O3 composite beads were achieved. XRD analysis confirmed the formation of a-TCP phase in all composite compositions. SEM investigations of the produced composite beads revealed that bimodal pore size distribution, fine and coarse, was achieved.
KEYWORDS:
Bovine Bone; Hydroxyapatite; Alumina; Bioceramic; Layered Composite; TCP (Tricalcium Phosphate)
Copy the following to cite this article:
AYDIN G, YILDIRIM K, KALEMTAS A. Naturally Derived Tricalcium Phosphate Based Porous Composite Bead Production. Mat. Sci. Res. India; 16 (3).
|
Copy the following to cite this URL:
AYDIN G, YILDIRIM K, KALEMTAS A. Naturally Derived Tricalcium Phosphate Based Porous Composite Bead Production. Mat. Sci. Res. India; 16 (3). Available from: https://bit.ly/36Nc4LY
|
Introduction
Accidents, falls, sports injuries or other causes such as low bone density and osteoporosis may lead to bone injuries/fracture.1 Implantation of bone autografts or allografts is considered an ideal strategy to heal bone defects. However, some drawbacks such as limited availability, extended surgical time and complications related to its harvesting process like donor site morbidity and pain limit the widespread usage of these strategies. Increasing advances in technology enabled researchers to develop synthetic, bioceramic, and polymer based bone substitute materials.2-5 Bioactive calcium phosphate (CP) ceramics such as hydroxyapatite (HA) and tricalcium phosphate (TCP) are attractive materials for bone tissue applications because of their similarity to natural bone, osteoconductivity, bioactivity, biocompatibility and chemical stability in body fluid.6, 7 HA and TCP ceramics are the most commonly used CP ceramics for bone replacement.
There are two main production methods for the calcium phosphate ceramic powders such as HA and TCP. These are relatively high cost conventional chemical methods.8-12 and relatively low-cost natural resources.8, 13-17 Natural sources such as bovine bones,18-23 fish scales,24-26 egg shells,27-29 fruit wastes,27 waste kina shells,30 sea snail,31 and oyster shells.32, 33 are used to produce micro and nano CP ceramic powder synthesis.
Despite excellent biocompatibility properties, low strength and brittle nature limits the use of HA [Ca10 (PO4)6(OH)2] especially in load-bearing applications.34, 35 HA decomposes into CP phases such as TCP and tetracalcium phosphate (TTCP). Low mechanical strength of HA is attributed to these calcium phosphate phases which are brittle and have weaker strength.36 Several strategies such as making composites and using different pressing/sintering methods like hot press sintering,37 spark plasma sintering,38, 39 underwater shock compaction.40 and microwave.41 have been employed to improve the mechanical properties of HA.
Reinforcing porous HA and TCP with materials such as alumina,42, 43 zirconia,43-45 titania.46, 47 and calcium silicate.48, 49 have been applied to enhance strength and fracture toughness of HA ceramics. Alumina is an attractive bio-inert material with high chemical and mechanical strength however it cannot form biochemical interfacial bond with the natural tissue.42, 50 On the other hand HA is a highly bioactive ceramic able to form a sound, firm bond with bone tissue. Therefore, highly bioactive composite scaffolds with a relatively enhanced mechanical strength can be produced by reinforcement of porous HA with alumina powder.51 The pores in the structure of these bioceramics provide enough space for cell ingrowth and nutrient uptake facilitating bone tissue formation.52 Due to these advantages porous materials are potentially suitable for various hard tissue applications such as bone cement, total hip joints and tooth implants.53-56
So far, several methods have been reported for the preparation of HA and TCP composites such as sol–gel process,57 chemical precipitation process,58 spark plasma sintering,59 and high energy ball milling.60 In this study, a facile innovative approach is applied to produce porous a-TCP-CeO2-Al2O3 composite beads via using bovine bone derived HA and alumina ceramics and pressureless sintering. Starch, an environmentally friendly and economic pore-forming agent, is used to achieve interconnected, highly open porosity containing composite beads. Starch also has a controlled quality, simple processing, commercial availability, easy handling and low burnout temperature without generation of toxic materials.61
Experimental Studies
Bovine bones, collected from a local meat shop in Konya/Turkey, were initially manually cleaned and then boiled in hot water to remove the adherent meat and other organics. Cleaned bones were rinsed with distilled water for many times and then dried in an oven at 100°C overnight before calcination.
Bovin bones were calcined at 950°C for 3 hours at a heating rate of 10°C/min in air atmosphere. After calcination bones were ground to the powder form in an agate mortar. Al2O3 (Esan Eczacibasi Group, Turkey) and CeO2 powders were used to produce three different a-TCP-Al2O3 composite beads (Table 1). Corn starch (Gunes, Turkey) was obtained from a local market and used as a pore forming agent. Sodium alginate (SA) and calcium chloride (CaCl2) were obtained from Katki Dunyasi, Turkey.
Table 1: Designed compositions
|
Designation
|
HA Amount, g
|
Al2O3 Amount, g
|
CeO2 Amount, g
|
Starch Amount, g
|
|
30H10A5C
|
30
|
10
|
5
|
10
|
|
20H20A5C
|
20
|
20
|
5
|
10
|
|
10H30A5C
|
10
|
30
|
5
|
10
|
H: HA, A: Al2O3, C: CeO2
Composite bead production was performed by the following method. SA was dissolved in distilled water by magnetic stirring at room temperature for 24 hours to achieve 3 wt.% SA solution. To prepare a homogenous slurry 45 g ceramic powders (HA + Al2O3 + CeO2), 10 g starch, and 150 mL SA solution was mixed in a planetary ball mill at a rotation speed of 300 rpm for 50 minutes. The cross-linking solution was prepared via dissolving 1 wt.% CaCl2 in distilled water by magnetic stirring at room temperature for an hour. Achieved aqueous solution was added dropwise into the CaCl2 aqueous solution at room temperature using a 60 mL hypodermic syringe through a needle under a constant stirring condition at room temperature. The alginate−ceramic composite beads are obtained via cross-linking in the CaCl2 solution for at least an hour. Subsequently the beads are removed from the CaCl2 solution by sieving and washed for many times with distilled water. Fig. 1 depicts the fabricated spherical ceramic−starch−alginate green composite beads.
Figure 1: Spherical ceramic−starch−alginate green composite beads. (a) 30H10A5C, (b) 20H20A5C, and (c) 10H30A5C.
The thermal behavior of the starch and sodium alginate was investigated via thermogravimetry (TG) analysis (TA, SDT650) in an air atmosphere at a heating rate of 10°C/min. Ceramic−starch−alginate composite beads were pressureless sintered at 1200°C for an hour. Applied heating and cooling rate was 10°C/minute. Porosity content, pore size and distribution, grain size and distribution of the porous composite beads were investigated with a scanning electron microscope (SEM, Carl Zeiss/Gemini 300) in a secondary electron (SE) image mode. Phase distribution and elemental compositions of the grains were investigated via an energy dispersive X-ray spectrometer (EDX, Bruker). Phase content investigation of the calcined bovin bones and produced composite beads were carried out by X-ray diffraction (XRD, Bruker AXS/Discovery D8), using monochromatic Cu-Kα radiation (l=1.5406Å).
Results and Discussion
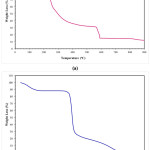
According to TG analysis, the corn starch demonstrated three significant weight change stages (Fig. 2-b), while five weight change reaction was occurred when sodium alginate was analyzed (Fig. 2-a). The first stage (room temperature-190°C) in the TGA thermogram was related to the release of the adsorbed water for both materials. At higher temperatures, decomposition of the materials was observed. Corn starch decomposed in two-stage, while sodium alginate decomposed in four stages. Sodium alginate loses almost 80 wt.% and 85wt% of its weight at 600°C and at 872°C, respectively. The corn starch loses approximately 100 wt.%. of its weight at 600°C. The corn starch undergoes decomposition suddenly in the beginning stage, which undergoes up to 326°C, and after this temperature, the weight loss speed was decreased.
Figure 2: TG analysis result of (a) alginate, and (b) corn starch.
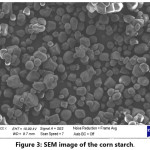
SEM investigations of the corn starch (Fig. 3) revealed that the particle size distribution of the starch is relatively narrow. Particle size of the starch is changing from ~5 to ~25 mm range.
Figure 3: SEM image of the corn starch.
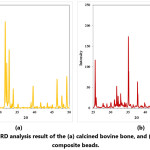
XRD analysis result of the calcined bovine bone and sintered composite beads are given in Fig. 4. XRD analysis revealed that only HA phase was existing in the calcined bovine bones. This result is consistent with the literature reporting that after calcination of bovine bones at 600-1000°C for 2-3 hours, the only stable phase is HA. [62, 63] XRD analysis result of the sintered composite beads revealed that HA phase is not existing in the composite beads. Hydroxyapatite is a thermally unstable material at high temperatures, and depending on its stoichiometry decomposes at 800-1200°C [44]. Sintering of the ceramic−starch−alginate green composite beads at 1200°C for 1 hour resulted in the decomposition of the hydroxyapatite phase and formation of a-TCP. XRD analysis revealed that a-TCP-CeO2-Al2O3 (Fig. 4) composite beads were achieved.
Figure 4: XRD analysis result of the (a) calcined bovine bone, and (b) sintered composite beads.
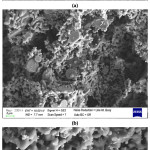
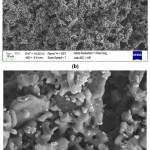
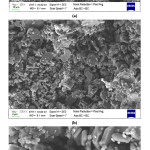
SEM investigations of the produced composite beads (Fig. 5-7) showed that highly interconnected and open porous structure was obtained for all compositions. It is also determined that bimodal pore size and distribution was achieved. Removal of sodium alginate and also relatively low pressureless sintering temperature resulted in small pores between the ceramic particles. Also, different morphologies of the starting materials enhanced the fine porosity development in the microstructure of the composite beads. On the other hand, removal of the corn starch from the structure during the sintering process resulted in the formation of big pores (~5-25 mm). SEM investigations showed that particle size of the corn starch was changing from ~5 to ~25 mm range (Fig. 3), and achieved porosity sizes are in good agreement with this finding. It was also observed that a good bonding was achieved between the ceramic particles during the sintering process via liquid phase sintering and neck formations took place.
Figure 5: SEM images of 20H20A5C sample at (a) 500X, (b) 2000X, and (c) 5000X.
Figure 6: SEM images of 30H10A5C sample at (a) 250X, (b) 500X, (c) 5000X, and (d) 10000X.
Figure 7: SEM images of 10H30A5C sample at (a) 500X, (b) 2000X, and (c) 5000X.
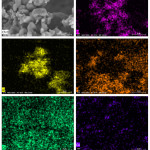
SEM image of sample 30H10A5C and SEM–EDX Ka maps of O, Al, P, Ca, and Ce are given in Fig. 8. Elemental distribution maps of the investigated sample confirmed that the produced composite beads are homogenously prepared.
Figure 8: SEM image of sample 30H10A5C and SEM–EDX Ka maps of O, Al, P, Ca, and Ce
Conclusions
- A simple, eco-friendly, and innovative approach is applied to produce porous a-TCP-CeO2-Al2O3 composite beads via using bovine bone-derived HA, CeO2, and Al2O3 as starting materials.
- Starch, an environmentally friendly and economic pore-forming agent, is used to achieve highly porous composite beads. Highly interconnected and open porous structure was obtained for all three compositions. It was also determined that a bimodal pore size and distribution was achieved.
- HA and TCP based porous beads are very essential materials, especially for bone tissue engineering applications. Due to low mechanical properties of CP ceramics, oxide reinforced CP ceramics can enlarge the applications of these materials due to better mechanical performances. Fabricated highly porous a-TCP-CeO2-Al2O3 composite beads are promising candidates for various biological and biomedical applications, as well as various applications such as adsorption of dyes, heavy metals, desalination, water treatment. Produced beads also can be used to reinforce different matrix materials to achieve innovative composite materials.
- These are the initial findings of the newly started research. New composition designs will be performed, and cytotoxicity, antibacterial and drug release properties of the produced spheres will be examined in the future studies.
Acknowledgment
The authors gratefully acknowledge Bursa Technical University Central Research Laboratory (Bursa, Turkey) for the TG, XRD, SEM, and SEM-EDX analysis. The authors also gratefully acknowledge Murat EROGLU for the XRD and SEM analysis.
Funding Source
This research received no specific grant from any funding agency.
Conflict of Interest
The authors declare that she/he has no conflict of interest regarding the publication of this article.
References
- A. Ibrahim, 3D bioprinting bone, 3D Bioprinting for Reconstructive Surgery, Elsevier2018, pp. 245-275.
- K. Arvidson, B. Abdallah, L. Applegate, N. Baldini, E. Cenni, E. Gomez‐Barrena, D. Granchi, M. Kassem, Y. Konttinen, K. Mustafa, Bone regeneration and stem cells, Journal of cellular and molecular medicine 15(4) (2011) 718-746.
- M. Yasaei, A. Zamanian, F. Moztarzadeh, M. Ghaffari, M. Mozafari, Characteristics improvement of calcium hydroxide dental cement by hydroxyapatite nanoparticles. Part 1: formulation and microstructure, Biotechnology and applied biochemistry 60(5) (2013) 502-509.
- M. Jafarkhani, A. Fazlali, F. Moztarzadeh, M. Mozafari, Mechanical and structural properties of polylactide/chitosan scaffolds reinforced with nano-calcium phosphate, Iranian Polymer Journal 21(10) (2012) 713-720.
- P. Holzmann, E. Niculescu-Morzsa, H. Zwickl, F. Halbwirth, M. Pichler, M. Matzner, F. Gottsauner-Wolf, S. Nehrer, Investigation of bone allografts representing different steps of the bone bank procedure via the CAM-model, ALTEX-Alternatives to animal experimentation 27(2) (2010) 97-103.
- B.O. Asimeng, Tensile properties, water absorption and enzymatic degradation studies of polyethylene/starch filled hydroxyapatite blend for orthopaedic applications, 2016.
- M. Mucalo, Animal-bone derived hydroxyapatite in biomedical applications, Hydroxyapatite (HAp) for biomedical applications, Elsevier2015, pp. 307-342.
- S. Mondal, G. Hoang, P. Manivasagan, M.S. Moorthy, H.H. Kim, T.T.V. Phan, J. Oh, Comparative characterization of biogenic and chemical synthesized hydroxyapatite biomaterials for potential biomedical application, Mater Chem Phys 228 (2019) 344-356.
- A. Permana, A. Utami, U. Handajani, H. Setyawati, Facile Sol-Gel Synthesis of Calcium Phosphates: Influence of Ca/P Ratio and Calcination Temperature, IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2019, p. 012001.
- S. Koutsopoulos, Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 62(4) (2002) 600-612.
- A.C. Tas, F. Korkusuz, M. Timucin, N. Akkas, An investigation of the chemical synthesis and high-temperature sintering behaviour of calcium hydroxyapatite (HA) and tricalcium phosphate (TCP) bioceramics, Journal of Materials Science: Materials in Medicine 8(2) (1997) 91-96.
- J. Duncan, J.F. MacDonald, J.V. Hanna, Y. Shirosaki, S. Hayakawa, A. Osaka, J.M. Skakle, I.R. Gibson, The role of the chemical composition of monetite on the synthesis and properties of α-tricalcium phosphate, Materials Science and Engineering: C 34 (2014) 123-129.
- C. Xu, M. Nasrollahzadeh, M. Selva, Z. Issaabadi, R. Luque, Waste-to-wealth: biowaste valorization into valuable bio (nano) materials, Chemical Society Reviews 48(18) (2019) 4791-4822.
- M. Akram, R. Ahmed, I. Shakir, W.A.W. Ibrahim, R. Hussain, Extracting hydroxyapatite and its precursors from natural resources, J Mater Sci 49(4) (2014) 1461-1475.
- G.R. Khayati, F. Ghanbari Rad, A Novel Approach for Direct Preparation of Hydroxyapatite Nanoparticles from natural source using Microwave, Journal of Ultrafine Grained and Nanostructured Materials 52(1) (2019) 110-121.
- L. Ozyegin, F. Sima, C. Ristoscu, I.A. Kiyici, I.N. Mihailescu, O. Meydanoglu, S. Agathopoulos, F. Oktar, Sea snail: an alternative source for nano-bioceramic production, Key Engineering Materials, Trans Tech Publ, 2012, pp. 781-786.
- S. Sánchez-Salcedo, M. Vila, A. Diaz, C. Acosta, I. Barton, A. Escobar, M. Vallet-Regí, Synthesis of HA/β-TCP bioceramic foams from natural products, J Sol-Gel Sci Techn 79(1) (2016) 160-166.
- H.L. Jaber, T.A. Kovács, Preparation and synthesis of hydroxyapatite bio-ceramic from bovine bone by thermal heat treatment, Építöanyag (Online) (3) (2019) 98-101.
- A. Niakan, S. Ramesh, P. Ganesan, C. Tan, J. Purbolaksono, H. Chandran, W. Teng, Sintering behaviour of natural porous hydroxyapatite derived from bovine bone, Ceram Int 41(2) (2015) 3024-3029.
- F. Miculescu, A. Maidaniuc, M. Miculescu, N. Dan Batalu, R. Cătălin Ciocoiu, S.t.I. Voicu, G.E. Stan, V.K. Thakur, Synthesis and characterization of jellified composites from bovine bone-derived hydroxyapatite and starch as precursors for robocasting, ACS omega 3(1) (2018) 1338-1349.
- H. Asgharzadeh Shirazi, M. Ayatollahi, B. Beigzadeh, Preparation and characterisation of hydroxyapatite derived from natural bovine bone and PMMA/BHA composite for biomedical applications, Mater Technol 31(8) (2016) 448-453.
- A. Maidaniuc, F. Miculescu, S.I. Voicu, C. Andronescu, M. Miculescu, E. Matei, A.C. Mocanu, I. Pencea, I. Csaki, T. Machedon-Pisu, Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles, Applied Surface Science 438 (2018) 158-166.
- S. Londoño-Restrepo, R. Jeronimo-Cruz, E. Rubio-Rosas, M. Rodriguez-García, The effect of cyclic heat treatment on the physicochemical properties of bio hydroxyapatite from bovine bone, Journal of Materials Science: Materials in Medicine 29(5) (2018) 52.
- P. Terzioğlu, H. Öğüt, A. Kalemtaş, Natural calcium phosphates from fish bones and their potential biomedical applications, Materials Science and Engineering: C 91 (2018) 899-911.
- C. Santana, C. Piccirillo, S. Pereira, R. Pullar, S. Lima, P. Castro, Employment of phosphate solubilising bacteria on fish scales–Turning food waste into an available phosphorus source, Journal of Environmental Chemical Engineering 7(5) (2019) 103403.
- C. Piccirillo, M. Silva, R. Pullar, I.B. Da Cruz, R. Jorge, M. Pintado, P.M. Castro, Extraction and characterisation of apatite-and tricalcium phosphate-based materials from cod fish bones, Materials Science and Engineering: C 33(1) (2013) 103-110.
- S.-C. Wu, H.-K. Tsou, H.-C. Hsu, S.-K. Hsu, S.-P. Liou, W.-F. Ho, A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite, Ceram Int 39(7) (2013) 8183-8188.
- I. Abdulrahman, H.I. Tijani, B.A. Mohammed, H. Saidu, H. Yusuf, M. Ndejiko Jibrin, S. Mohammed, From garbage to biomaterials: an overview on egg shell based hydroxyapatite, Journal of Materials 2014 (2014).
- A. Arabhosseini, Application of eggshell wastes as valuable and utilizable products: A review, Research in Agricultural Engineering 64(2) (2018) 104-114.
- A. Shavandi, V. Wilton, A.E.-D.A. Bekhit, Synthesis of macro and micro porous hydroxyapatite (HA) structure from waste kina (Evechinus chloroticus) shells, Journal of the Taiwan Institute of Chemical Engineers 65 (2016) 437-443.
- O. Gunduz, Y. Sahin, S. Agathopoulos, B. Ben-Nissan, F.N. Oktar, A new method for fabrication of nanohydroxyapatite and TCP from the sea snail Cerithium vulgatum, J Nanomater 2014 (2014) 1.
- S.-C. Wu, H.-C. Hsu, S.-K. Hsu, C.-P. Tseng, W.-F. Ho, Effects of calcination on synthesis of hydroxyapatite derived from oyster shell powders, Journal of the Australian Ceramic Society (2019) 1-8.
- S.-C. Wu, H.-C. Hsu, S.-K. Hsu, C.-P. Tseng, W.-F. Ho, Preparation and characterization of hydroxyapatite synthesized from oyster shell powders, Advanced Powder Technology 28(4) (2017) 1154-1158.
- M. Aminzare, A. Eskandari, M. Baroonian, A. Berenov, Z.R. Hesabi, M. Taheri, S. Sadrnezhaad, Hydroxyapatite nanocomposites: Synthesis, sintering and mechanical properties, Ceram Int 39(3) (2013) 2197-2206.
- H. Gheisari, E. Karamian, M. Abdellahi, A novel hydroxyapatite–hardystonite nanocomposite ceramic, Ceram Int 41(4) (2015) 5967-5975.
- I. Mobasherpour, M.S. Hashjin, S.R. Toosi, R.D. Kamachali, Effect of the addition ZrO2–Al2O3 on nanocrystalline hydroxyapatite bending strength and fracture toughness, Ceram Int 35(4) (2009) 1569-1574.
- Z. Xihua, L. Changxia, L. Musen, B. Yunqiang, S. Junlong, Fabrication of hydroxyapatite/diopside/alumina composites by hot-press sintering process, Ceram Int 35(5) (2009) 1969-1973.
- W. Que, K.A. Khor, J. Xu, L. Yu, Hydroxyapatite/titania nanocomposites derived by combining high-energy ball milling with spark plasma sintering processes, Journal of the European Ceramic Society 28(16) (2008) 3083-3090.
- A.A. Chaudhry, H. Yan, K. Gong, F. Inam, G. Viola, M.J. Reece, J.B. Goodall, I. ur Rehman, F.K. McNeil-Watson, J.C. Corbett, High-strength nanograined and translucent hydroxyapatite monoliths via continuous hydrothermal synthesis and optimized spark plasma sintering, Acta biomaterialia 7(2) (2011) 791-799.
- A. Chiba, S. Kimura, K. Raghukandan, Y. Morizono, Effect of alumina addition on hydroxyapatite biocomposites fabricated by underwater-shock compaction, Materials Science and Engineering: A 350(1-2) (2003) 179-183.
- D.J. Curran, T.J. Fleming, M.R. Towler, S. Hampshire, Mechanical parameters of strontium doped hydroxyapatite sintered using microwave and conventional methods, Journal of the mechanical behavior of biomedical materials 4(8) (2011) 2063-2073.
- J. Teixeira, J. Alburquerque, E. Duarte, S. Silva, R. Nogueira, In vitro drug release study from hydroxyapatite-alumina composites, J Sol-Gel Sci Techn 89(2) (2019) 521-530.
- M. Es-Saddik, S. Laasri, M. Taha, A. Laghzizil, A. Hajjaji, Finite Element Modeling of Mechanical Behavior of Al2O3–ZrO2 Reinforced Calcium Phosphate Biomaterials, Sensor Letters 16(6) (2018) 478-483.
- K. Castkova, H. Hadraba, A. Matousek, P. Roupcova, Z. Chlup, L. Novotna, J. Cihlar, Synthesis of Ca, Y-zirconia/hydroxyapatite nanoparticles and composites, Journal of the European Ceramic Society 36(12) (2016) 2903-2912.
- Y. Cao, T. Shi, C. Jiao, H. Liang, R. Chen, Z. Tian, A. Zou, Y. Yang, Z. Wei, C. Wang, Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing, Ceram Int 46(2) (2020) 2300-2308.
- R. Khattab, H. Badr, M. Zawrah, Effect of processing techniques on properties of porous TiO2 and TiO2/hydroxyapatite composites, Ceram Int 44(7) (2018) 8643-8649.
- Q. Yuan, L. He, Z.-J. Qian, C. Zhou, P. Hong, Z. Wang, Y. Wang, S. Sun, C. Li, Significantly Accelerated Osteoblast Cell Growth on TiO2/SrHA Composite Mediated by Phenolic Compounds (BHM) from Hippocamp us kuda Bleeler, Acs Appl Mater Inter 10(36) (2018) 30214-30226.
- D.J. Lee, J. Kwon, Y.I. Kim, X. Wang, T.J. Wu, Y.T. Lee, S. Kim, P. Miguez, C.C. Ko, Effect of pore size in bone regeneration using polydopamine‐laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology, Orthodontics & craniofacial research 22 (2019) 127-133.
- A.A. Oshkour, S. Pramanik, M. Mehrali, Y.H. Yau, F. Tarlochan, N.A.A. Osman, Mechanical and physical behavior of newly developed functionally graded materials and composites of stainless steel 316L with calcium silicate and hydroxyapatite, journal of the mechanical behavior of biomedical materials 49 (2015) 321-331.
- S.M.H. Ghazanfari, A. Zamanian, Phase transformation, microstructural and mechanical properties of hydroxyapatite/alumina nanocomposite scaffolds produced by freeze casting, Ceram Int 39(8) (2013) 9835-9844.
- A. Yelten, S. Yilmaz, F.N. Oktar, Sol–gel derived alumina–hydroxyapatite–tricalcium phosphate porous composite powders, Ceram Int 38(4) (2012) 2659-2665.
- L.L. Hench, Bioceramics: from concept to clinic, Journal of the american ceramic society 74(7) (1991) 1487-1510.
- C. Piconi, G. Maccauro, F. Muratori, E.B. Del Prever, Alumina and zirconia ceramics in joint replacements, Journal of Applied Biomaterials and Biomechanics 1(1) (2003) 19-32.
- A. Marti, Inert bioceramics (Al2O3, ZrO2) for medical application, Injury 31 (2000) D33-D36.
- J. Chakraborty, D. Basu, Bioceramics—a new era, Transactions of the Indian Ceramic Society 64(4) (2005) 171-192.
- T. Thamaraiselvi, S. Rajeswari, Biological evaluation of bioceramic materials-a review, Carbon 24(31) (2004) 172.
- J. Chen, Y. Wang, X. Chen, L. Ren, C. Lai, W. He, Q. Zhang, A simple sol-gel technique for synthesis of nanostructured hydroxyapatite, tricalcium phosphate and biphasic powders, Materials Letters 65(12) (2011) 1923-1926.
- Y.-M. Sung, J.-C. Lee, J.-W. Yang, Crystallization and sintering characteristics of chemically precipitated hydroxyapatite nanopowder, Journal of crystal growth 262(1-4) (2004) 467-472.
- S. Klébert, C. Balázsi, K. Balázsi, E. Bódis, P. Fazekas, A.M. Keszler, J. Szépvölgyi, Z. Károly, Spark plasma sintering of graphene reinforced hydroxyapatite composites, Ceram Int 41(3) (2015) 3647-3652.
- T.R. Tadjiev, S.S. Chun, S.Y. Kim, Mechano-chemical synthesis of biphasic calcium phosphates with the various ratio of HA and β-TCP, Key Engineering Materials, Trans Tech Publ, 2007, pp. 7-10.
- A. Kalemtas, G. Topates, H. Özcoban, H. Mandal, F. Kara, R. Janssen, Mechanical characterization of highly porous β-Si3N4 ceramics fabricated via partial sintering & starch addition, Journal of the European Ceramic Society 33(9) (2013) 1507-1515.
- N. Bano, S.S. Jikan, H. Basri, S. Adzila, D.M. Zago, XRD and FTIR study of A&B type carbonated hydroxyapatite extracted from bovine bone, AIP Conference Proceedings, AIP Publishing, 2019, p. 020100.
- S. Ramesh, Z.Z. Loo, C.Y. Tan, W.K. Chew, Y.C. Ching, F. Tarlochan, H. Chandran, S. Krishnasamy, L.T. Bang, A.A. Sarhan, Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications, Ceram Int 44(9) (2018) 10525-10530.

This work is licensed under a Creative Commons Attribution 4.0 International License.


 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal