Preparation and Study of Corrosion Behavior of Some Simulated Radioactive Waste Glasses
Patit Paban Malik
Department of Chemistry, B.B. College, Asansol, West Bengal, India
Corresponding Author E-mail: patitbu99@rediffmail.com
DOI : http://dx.doi.org/10.13005/msri/160310
Article Publishing History
Article Received on : 09/11/2019
Article Accepted on : 03/12/2019
Article Published : 11 Dec 2019
Plagiarism Check: Yes
Reviewed by: Goutam Hazra
Second Review by: Uday Kiran Final Approval by: Subhasis Roy
Final Approval by: Subhasis Roy
Article Metrics
ABSTRACT:
To safe environment from radioactive waste it is important to fix them as radioactive waste glasses. The corrosion behavior of radioactive waste glasses in water is significantly important. Radionuclides return to the biosphere by means of leaching from waste form into ground water. Finally the ground water containing the radionuclide are transported to the surface. In this study, the preparation, characterization and leaching behavior of some borosilicate (BS) and lead iron phosphate (LIP) of different chemical composition doped with simulated nuclear waste oxide were investigated. We measured the pH found to be in the range from 6.78 up to 7.79 of the leachate solution at normal temperature and at varying time intervals. Leaching study of these glasses were conducted with the help of Soxhlet distillation apparatus with distilled water upto 24 hours and for BS9 - BS12 upto 100 hours duration. Weight losses were are measured with respect to time of leaching. Leach rate of some borosilicate glass samples loaded with uranium are calculated from surface area measurements. The results are reported in the range 1.34x10-4 g.m-2.hr-1 and 6.26 x 10-4 g.m-2.hr-1 respectively at 90°C.
KEYWORDS:
Nuclear Wastes; Corrosion; Leaching; Fission Fragments; Glass Formers and Modifiers
Copy the following to cite this article:
Malik P. P. Preparation and Study of Corrosion Behavior of Some Simulated Radioactive Waste Glasses. Mat. Sci. Res. India; 16 (3).
|
Copy the following to cite this URL:
Malik P. P. Preparation and Study of Corrosion Behavior of Some Simulated Radioactive Waste Glasses. Mat. Sci. Res. India; 16 (3). Available from: https://bit.ly/2PMFrXZ
|
Introduction
The threaten problem of global warming and climate change have allowed the benefits of nuclear power such as non-production of greenhouse gases to be recognized. The future nuclear energy depends largely on the management of radioactive waste generated at different steps of the nuclear fuel cycle. Waste disposal expert (mainly radioactive waste) all over the world believes that ultimate disposal of high level radioactive waste to immobilize them to solid matrix, preferably glass. Glass can dissolve most of the elements present in the periodic table and it can be used as a medium for waste containment.1,2 Thus the fission product becomes a constituent of the glass structure. Glass is a truly ‘secular’ matrix and in which all the elements become part and parcel of the glass containing the waste. It is difficult to dissolve glass in water hence it is high leach resistance. Low leaching property is one of the most desirable criteria for the solid matrix to vitrify the radioactive waste. The less corrosion tendency will select the expected radioactive waste glass. Borosilicate and lead iron phosphate (LIP) glasses are considered as a suitable solid matrix for high level radioactive waste.3,4 The final disposal of high level radioactive waste involves multi- barrier approach. Radionuclide from the radioactive waste glass may enter into the biosphere, when ground water comes in contact with the radioactive waste glass after penetrating all the barrier applied for the radioactive waste glass. Corrosion behavior of radioactive waste glass is often used to describe as the resistance of leaching in aqueous medium.5 So leaching study of radioactive waste glasses are important because they are to be stored as geological repositories for long period. It is difficult to measure the long term corrosion directly, but with the help of short –term laboratory experiments, long term corrosion can be estimated.
The corrosion mechanism of borosilicate nuclear waste glass in water can be understood at the present time. Glass dissolution is the summation of various processes involving different mechanism. At first hydration of glass is taking place by diffusion water into the glass network and then by ion exchange between positive protons exists in the ground water and alkali metal in the glass formation. Another way by which dissolution of glasses takes place is hydrolysis of the bond, ionic – covalent bond are the most soluble elements of the glass net work.
In the work, borosilicate and lead iron phosphate glasses of different composition loaded with simulated radioactive waste were studied. The chemical corrosion of these glasses in distilled water were measured in terms of leaching study.6-8 The aim of the present work is to find out composition of borosilicate and LIP glasses with high chemical durability because this is one of the most desirable characteristic to the reliability of vitrified nuclear waste products stored in their final deep geological repositories.9,10 Another key factor to be investigated is the low melting of the glass as some amount of Ru and Cs activity escape from the glasses at higher temperature.12-14
Materials and Method
To prepare the glass batches we used in ingredients like quartz powder (AR grade, Oxford laboratory reagent) as source of silica, Fe2O3 (AR Grade, Dipak laboratories), PbO (AR grade, Dipak laboratories), Borax (AR grade, RANKEM), CeO2 (AR grade, Hi Media), SrO (AR grade, Aldrich), BaO (AR Grade, Boroyne), P2O5 (AR grade BDH), uranyl acetate (AR grade, BDH). Glass batches in the borosilicate and LIP systems are synthesized in acetone medium on the basis of some review work and corresponding ternary diagrams of predetermined compositions. The ingredients were well mixed with the help of mortar –vessel and dried up. It is then taken in a alumina crucibles and melted in a programmable muffle furnace in the range 8000 C – 950°C and soaked for the periods of 30 min – 90 min in air to achieve homogeneous mixture. At the time of melting operation we monitor both time and temperature of melting. The composition having lower the glass processing temperature will be the better glass for incorporation of nuclear waste with high volatility like some of the fission fragment (RuO). The oxides SiO2 and B2O3(borax) act as the glass former while BaO, SrO, CeO2 and Fe2O3 act as modifiers, and PbO act as intermediates. The effect of different modifier ions like Pb2+ , Ba2+, Na+, Fe3+, Al3+, Ce4+ and Sr2+ on the melting points and time of melting is quite evident. The compositions used in the present study are shown in Table 1. Some of the borosilicate glasses [BS9 – BS 12] were prepared by taking uranyl acetate directly into itself since U is α/β active and by measuring the net counts per sec (cps), the leaching property can be determined with the help of a Geiger-Muller counter by ‘radiotracer technique’.
Table 1: Different Glass Compositions prepared (wt %)
|
Glass
|
SiO2
|
Na2 B4O7
|
PbO
|
BaO
|
Fe2O3
|
Al2O3
|
P2O5
|
SrO
|
Uranyl aceatate
|
|
BS7
|
40
|
24
|
–
|
–
|
13
|
3
|
–
|
20
|
–
|
|
BS8
|
35
|
24
|
–
|
–
|
13
|
3
|
–
|
25
|
|
|
BS9
|
39.6
|
20
|
30
|
5
|
–
|
–
|
–
|
–
|
5.4
|
|
BS10
|
39.0
|
20
|
30
|
5
|
–
|
–
|
–
|
–
|
6.0
|
|
BS11
|
39.0
|
20
|
29.5
|
5.0
|
–
|
–
|
–
|
–
|
6.5
|
|
BS12
|
39.0
|
20
|
25
|
5
|
–
|
–
|
–
|
5
|
6.0
|
|
LIP5
|
–
|
–
|
49
|
10(CeO2)
|
8
|
–
|
33
|
–
|
–
|
|
LIP6
|
–
|
–
|
49
|
5 (CeO2)
|
8
|
–
|
33
|
5
|
–
|
|
LIP7
|
–
|
–
|
52.4
|
–
|
8
|
–
|
33.1
|
–
|
6.5
|
|
LIP8
|
–
|
–
|
51.8
|
–
|
8
|
–
|
33.2
|
–
|
7.0
|
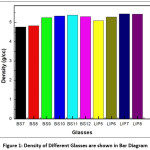
The densities of different glasses were measured by Archimedes methods. In this method water was used as immersion liquid and a hydrostatic balanced was used. For each sample, three consecutive measure were made and the results are the mean value.
From X-ray diffraction study it is cleared that the prepared sample are amorphous in nature. The respective temperature of melting and time of melting are shown in Table 2.
Table 2: Melting Temperature (°C), Time of Melting and density of different glass compositions
|
Glasses
|
M. P. (± 2oC)
|
Time(min)
|
Density(g/cm3)
|
|
BS7
|
900
|
30
|
4.7613
|
|
BS8
|
850
|
60
|
4.8231
|
|
BS9
|
800
|
90
|
5.2413
|
|
BS10
|
850
|
90
|
5.3241
|
|
BS11
|
900
|
90
|
5.3725
|
|
BS12
|
800
|
90
|
5.2939
|
|
LIP5
|
900
|
90
|
5.0825
|
|
LIP6
|
900
|
90
|
5.2738
|
|
LIP7
|
950
|
90
|
5.4327
|
|
LIP8
|
850
|
90
|
5.4238
|
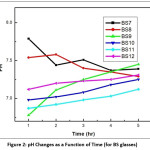
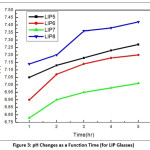
To determine the pH of the leachate solution, the glass sample was grinded and mess through to achieve 0.300 – 0.425 mm size. Then one (1) gm of the specified sized glass powder was taken in a beaker containing 40 ml distilled water. It was stirred with the help of a magnetic stirrer for few minutes. Systronics digital pH meter (Model 335) was used to determine the pH of the leachate solution and it was carried out at regular intervals of 1 hr., 2 hr., 3 hr., 4 hr. and 5 hr. respectively. In each case the mixture was stirred for 15 minutes. The leachate were extracted after each operation of leaching for Uranium containing glasses and then subjected to pH measurement for varying time intervals. The results are shown in table 3.
Table 3: pH of leachate after different time intervals
|
Glasses
|
PH in different time Time
|
|
|
1 hr.
|
2 hr.
|
3 hr.
|
4 hr.
|
5 hr.
|
|
BS7
|
7.79
|
7.44
|
7.51
|
7.37
|
7.39
|
|
BS8
|
7.54
|
7.58
|
7.40
|
7.35
|
7.29
|
|
BS9
|
6.78
|
7.11
|
7.25
|
7.35
|
7.45
|
|
BS10
|
6.98
|
7.02
|
7.08
|
7.18
|
7.25
|
|
BS11
BS12
|
6.87
7.12
|
6.92
7.20
|
6.98
7.23
|
7.03
7.25
|
7.12
7.31
|
|
LIP5
|
7.05
|
7.13
|
7.18
|
7.23
|
7.27
|
|
LIP6
|
6.90
|
7.07
|
7.14
|
7.18
|
7.20
|
|
LIP7
|
6.78
|
6.90
|
6.95
|
6.98
|
7.01
|
|
LIP8
|
7.14
|
7.20
|
7.36
|
7.38
|
7.42
|
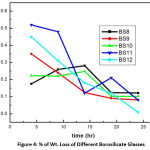
To study the leaching property about 0.5 gm of glass sample of the specified sized glass (0.300-0.425 mm) were accurately weighed and was taken in a nylon net through which glass powder did not pass out. A nylon net containing the sample was placed in a Soxhlet apparatus fitted with condenser and with a round bottom flask (500 ml capacity). Distilled water was used leaching medium and continued for varying period up to 24 hr and for some cases it was upto 100 hr. The heating being done by a heating mantle. [ASTM: C1285-02(2008)].15 The results are shown in table 4. For the borosilicate system Percentage of wt. loss vs time (hrs) plot is shown in fig. 4 and the same for lead iron phosphate glasses are shown in fig 5.
Table 4: Percentage of weight loss of glasses at different time intervals
|
% Wt. loss of glasses
|
Time
|
|
|
4hr
|
9 hr
|
14 hr
|
19 hr
|
24 hr
|
|
BS7
|
0.23
|
0.84
|
1.4
|
0.57
|
0.88
|
|
BS8
|
0.175
|
0.259
|
0.281
|
0.123
|
0.121
|
|
BS9
|
0.35
|
0.24
|
0.124
|
0.09
|
0.08
|
|
BS10
|
0.223
|
0.220
|
0.248
|
0.102
|
0.102
|
|
BS11
|
0.52
|
0.48
|
0.12
|
0.21
|
0.08
|
|
BS12
|
0.45
|
0.31
|
0.18
|
0.12
|
0.01
|
|
LIP5
|
0.14
|
0.10
|
0.02
|
0.01
|
0.00
|
|
LIP6
|
0.23
|
0.15
|
0.06
|
0.02
|
0.01
|
|
LIP7
|
0.13
|
0.21
|
0.15
|
0.08
|
0.00
|
|
LIP8
|
0.42
|
0.36
|
0.24
|
0.20
|
0.02
|
Statements that serve as captions for the entire table do not need footnote letters.
Figure 1: Density of Different Glasses are shown in Bar Diagram
Figure 2: pH Changes as a Function of Time (for BS glasses)
Figure 3: pH Changes as a Function Time (for LIP Glasses)
Figure 4: % of Wt. Loss of Different Borosilicate Glasses
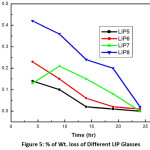
Figure 5: % of Wt. loss of Different LIP Glasses
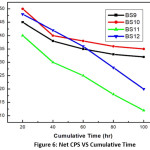
Figure 6: Net CPS VS Cumulative Time
Results and Discussion
The melting temperature and soaking periods of different glass compositions obtained in the present work was shown in table 2. From the X-ray diffraction study it was seen that the products to are amorphous. We monitor the temperature and time of melting as they are the principal factors, properties of the glass depends on these key factors. We are able to melt the glass at temperature (800-950oC) with a soaking period of 30 min – 90 min. These temperatures are much lower than earlier reported to be melted not below 10000 C.16,17 The different modifier ions play a important role in the variation of melting points and the exact trend being difficult to speculate. The effect of different modifier ions like Ba2+, Pb2+, Fe3+ and Sr2+ is quite evident on the melting points and time of melting.
Figure 2 & 3 show the result of pH study. In these figure plot of (pH vs time) different glass systems are shown. The curve for BS11 shows lowest value of pH and BS7 has highest pH value among the borosilicate glasses. The increasing tendency in the pH values aroused due to the mixed oxide effect of borosilicate system as well as in the case of LIP systems. The pH values obtained in this study are in good agreement with the equilibrium pH values according to their composition. To explain the result we may consider the following equilibrium in the aqueous system:
H 2O H + + OH−
The forward or the reverse direction of the equilibrium pH is controlled by the different modifier ions present in the glass released when the glass sample leaching out.18,19,20 The ionic radii (Å) of the different modifier ions are Pb2+ (1.33), Ba2+ (1.35), Fe3+ (0.69), Ce4+ (1.18) and Sr4+ (1.13). The ionic potential increases as following order Ba2+ (1.48) < Pb2+ (1.5) < Sr4+ (1.54) < Ce4+< (3.39) Fe3+ (4.69). A metal ion having greater ionic potential will attract more OH– ions to form the corresponding hydroxides M(OH)n. The formation of hydroxide ion increases the dissociation of H2O in the forward direction, releases more number of H+ ion in the medium.21 The resulting solution becomes acidic giving rise to low ph value. The BS10 glass contains uranyl acetate (6.0 wt%). Uranium present in the waste glass can act as a very good glass former and decreases the leaching tendency of the ions and hence the pH value goes out at a value just below 6.98.
Leaching property was studied with the help of Soxhlet distillation apparatus. For glasses not containing uranium, the results of % wt. losses at each stage (time in hr.) were taken. The plot of % of wt. loss vs time is shown in fig 4 & 5. The ‘radiotracer technique’ (cps vs. cumulative time) was applied for uranium containing glasses along with wt. Loss data. Figure 6 shows the corresponding plots. From the plot it is observed that with increasing cumulative time, the net cps (alpha or beta) has decreased in an exponential way. This signifies that with increasing leaching time, the release of uranium along with other constituents becomes slower and slower until getting stopped. This indicates that the glass becomes resistant to leaching and is a better choice for the disposal of nuclear waste compared. In case of BS10 -BS12 glasses, a similar type of plot is obtained.
The leach rates (LR) for BS9 and BS10 are determined from surface area analysis after measuring the leaching. These values are obtained as 1.34×10-4 g.m-2.hr-1 and 6.26 x 10-4 g.m-2.hr-1 respectively. The leach rate (LR) were measured from weight loss according to the relationship
LR =(mi – mf)/(SA x t)
where mi and mf refers the initial and final sample weights respectively, SA indicates the surface area of the sample and exposed to the leachant time is t. These rates are lower as compared to other system and thus Borosilicate glasses have much improved chemical durability.22,23,24 The % wt. loss of the radioactive waste significantly decreases after long period of time of leaching and finally die down with extent time.
Conclusion
In our present study, we are able to melt the glass composition at much lower temperature (800-9500C) for a soaking period of 30 min. to 90 min. This is one of the important side of our result (earlier reported ~10000C). It is the modifier ions which play a important role in lowering the melting temperature. The bond energy difference and the number of glass formers ions contributed the difference in melting point of systems. Lead can act as a good modifier and it improves the most desirable leach resistance property. When iron is added to lead phosphate glass, chemical durability of the glass is increased and also suppress the tendency for crystallization. The alkaline earth cations like Ba, Sr act as good modifiers and they break the Si-O-Si bridges by introducing of non-bridging O sites. Lead oxide has the capability to decrease the non bridging oxygen which makes the glass more durable. Ion-exchange with H3O+ is difficult for lead containing glasses which makes the glass more corrosion resistance. Again, between the glasses BS7 and BS9, BaO is present in the BS9 which is more electro positive than SrO which is present in BS7. In this study, the PH value measured from different leachate solution can be explained in terms of ionic potential, ionic radii and ionic size of different the modifier ions present in the glass network. These also can be explained in terms of surface layer hydration of the glass sample.
Borosilicate and LIP glasses can be used as potential solid matrix for immobilization of high level radioactive waste. This fixation of radionuclide is permanent and irreversible and will be the better choice for radioactive waste disposal. The radioactive waste glass with composition of lead iron phosphate showed that the corrosion rate is less than that of other glasses. The chemical durability of the glass increases with the addition of iron and lead to the glass composition. The chemical durability and the leachability properties of the glass are most important parameter of the radioactive waste from the view point of the environment. So the durability and the chemical resistance properties are improved with addition of different modifier ions. The study shows that the effect of alkaline earth oxides BaO and SrO quite significance and it decrease the durability while PbO improves the corrosion resistance properties. Another important result of the study is the low melting temperature of glasses which significantly reduces the volatilization of ruthenium and caesium.
Acknowledgement
The authors are very much thankful to Dr. J. Mukherjee and Dr. A. S. Sanyal, retired Scientist, CGCRI, for sharing their expertise and valuable guidance is gratefully acknowledged. The authors are thankful to UGC for financial assistance (Minor Research ProjectPSW-010/15-16).
Funding Source
The paper is funded by UGC vide (Minor Research ProjectPSW-010/15-16).
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- J. Mukerji and A. S. Sanyal ”Historical series: Indian work on vitreous matrices for the containment of radioactive waste 1960-1980”, Glass Technol., vol 45, no 3,pp 117-25, 2004
- S. V. Raman, “The effect of mixed modifiers on nuclear waste glass processing, leaching and Raman spectra”, J. Mater. Res., Vol. 13, No. 1, Jan 1998
- A. S. Pankou, O. G. Batyukhova, M. I. Ojoven and W. E. Lee, “Simulation of Self-Irradiation of High-sodium content Nuclear Waste Glasses”. Mater Res. Soc. Symp. Proc. Vol. 985, 2007.
- Jain, V., & Pan, Y.M. (2000). High-Level waste glass dissolution in simulated internal waste package environments. Scientific research on the back-end of the fuel cycle for the 21 century, (p. 575). France
- T. Yanagi, M. Yoshizoe and N. Nakatsuka, “Leach Rates of Lead-Iron Phosphate Glass Waste Forms”, Department of Nuclear Engineering, Faculty of Engineering, Osaka University WM’02 Conference, February, 24-28, 2002, Tucson, AZ1 16 .
- V. Petitjean, C. Fillet, R. Boen, C. Veyer, T. Flament “Development of Vitrification Process and Glass Formulation for Nuclear Waste Conditioning” WM’02 Conference, February 24-28, 2002.
- P.P.Malik, P.Mitra, T.Das, “Effect of Different Modifiers on Melting Points and pH under Leaching in Nuclear Waste Glasses in Borosilicate and Phosphate Systems”, Material Science Research India Vol. 9(1), pp105-109 (2012).
- Delbert E. Day (PI),Chandra S. Ray,Cheol-Woon Kim,et.a “Iron Phosphate Glasses: An Alternative for Vitrifying Certain Nuclear Wastes” l. Annual Report for DOE FG07-96ER45618, 2001-2002.
- M. J. Plodinec, “Vitrification chemistry and nuclear waste”, Non-Cryst Solids 8,pp 206-214, 1986 .
- M. J. Plodinec, “Borosilicate Glasses for nuclear waste immobilisation”, Glass Technology, vol 41, no 6, pp186-192 ,2000
- M. K. T. Nair, V. Venugopal, B.S. Kumar and D. D. Sood , “New Approaches in High Level Radioactive Waste Management”, In Proc. Nuclear & Radio Chemistry Symp., Andhra University December, pp 41-44,1992
- H Doweidar, Y. M Moustafa., K. El-Egili., I. Abbas, Vibrational Spectros., 37: 91 (2007).
- R. K. Brow, D. R. Tallant, S. T. Myer, C. C. Phifer, J. Non-Cryst. Solids, 191: 45 (1995).
- S. T. Reis, J. R. Martinell, “Cs immobilization by sintered lead iron phosphate glasses”; Journal of Non-Crystalline Solids, Volume 247, Issues 1-3, pp 241-247, 1999
- V.S. Thoral R.K.Mishra, S.Kumaran, A.K.Tyagi, Corrosion of Borosilicate Glasses Subjected to Aggressive Test Conditions: Structural Investigations “,Journal of Americam Ceramics Society, (2016)
- A. Bohre, K. Avasthi, V. I. Petkov, “Vitreous and crystalline phosphate high level waste matrices”, Journal of Industrial and Engineering Chemistry, (Feb,2017)
- Amrita Dhara, R.K. Mishra, R. Shukla, T.P. Valsala, V. Sudarsan, A.K. Tyagi, C.P. Kaushik, A comparative study on the structural aspects of sodium borosilicate glasses and barium borosilicate glasses: Effect of Al2O3 addition, Journal of Non-Crystalline Solids, Volume 447, 2016, Pages 283-289
- J. Kamizono, S. Hayakawa, S. Muraoka, J. Materials Science Letters,vol 10, pp423-425 , 1993.
- A. S. Sanyal, J. Mukerji, “Fixation of High Level Atomic Waste in Glass for Ultimate Disposal: Part II-Development of Vitreous Matrices for Containment of CIRUS, Tarapur & Ranapratap Sagar Nuclear Fuel Reprocessing Wastes”, J. Scientific & Industrial Research,vol 33, no9, pp436-460 , 1974.
- S. Jana, B. Karmakar and P.Kundu; “Unusual visible absorption in high PbO lead borate glass”, Matt.Sc. of Poland,Vol. 25, No.4, pp.1127-1134 , 2007.
- D.E. Day, Z. Wu, C. Ray, “Chemically Durable Glasses for Vitrifying Phosphate Containing Simulated Nuclear Wastes” Final Technical Report, Northwest National Laboratories, 1995.
- G. Hazra, A. Ghosh, T. das, P. Mitra, ”Preparation and studies of uranium doped lead iron phosphate simulated nuclear waste glasses”, International journal of Advanced Research in Engg. And Applied science,vol3, no 8,pp16-34,2014.
- K. Adachiand, H.Kuno,“Decomposition of ruthenium oxides in lead borosilicate glass,” Journal of the American Ceramic Society, vol.80, p.1055,1997.
- D. Banerjee, V.K. Sudarsan, A. Joseph, R.K. Mishra, I. Singh, P.K. Wattal, D. Das, J. Am. Ceram. Soc. 93(10) (2010) 3252.

This work is licensed under a Creative Commons Attribution 4.0 International License.

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal