Synthesis, Characterization and Methyl Orange Degradation Activity of Ti-Al Oxides Nanomaterial
Introduction
Various water treatment practices such as sedimentation–flocculation, coagulation, molecular sieving, ion exchange, reverse osmosis, membrane filtration, ozonation, chlorination, and chemical precipitation, have been utilized to remove pollutants. However, the application of combined adsorption-photocatalysis technique was found to accomplish complete degradation of pollutants into the water, gas and mineral forms.1 The metal oxides such as titanium oxide (TiO2), zinc oxide (ZnO), hematite (α-Fe2O3), maghemite (g – Fe2O3), magnetite (Fe3O4), manganese (III) oxide (Mn2O3), and manganese dioxide (MnO2) have recently gained significant attention for both adsorption and photocatalytic applications due to their superior thermal stability, corrosion resistance, and high surface area to volume ratio.2
TiO2 exhibits outstanding properties such as strong oxidizing power under ultraviolet irradiation, physicochemical properties, stability, and less toxicity. However, with its low surface area, rapid electron-hole (e–-h+) recombination, and agglomeration due to the presence of metal oxygen bond (M-O-M), the photocatalytic effectiveness of bare TiO2 was found to get seriously affected.3 To prevent these e–-h+ recombination and agglomerations, number of attempts were made in the past which includes the formation of nanocomposites,4 doping,5 noble and non-noble deposition6,7 and formation of heterojunction with narrow bandgap metal oxides. Among those modifications, the formation of a heterojunction with metal oxides that have high specific surface area becomes relatively the best technique. Furthermore, the adjustable pore size behavior and high stability of Al2O3 makes it superior support for TiO2.8
In addition, many researchers reported wide variety of materials such as mesoporous Al2O3@TiO2 nanocomposites synthesized by precipitation method for decolorization of reactive black 5 dye,2 two-phase TiO2/Al2O3 nanocomposite synthesized by hydrothermal used for adsorption and photocatalytic degradation of reactive brilliant red X-3B dye,9 mesoporous Al2O3–TiO2 nanocomposites synthesized by sol-gel method for photocatalytic degradation of imazapyr herbicide,10 TiO2/Al2O3 catalyst via sol-gel route using polymer templates for congo red degradation,11 TiO2/Al2O3 photocatalyst made by sol-gel method for photocatalytic oxidation of NO12 and NOx reduction.13,14 The obtained results have confirmed the positive effects of incorporation of Al2O3 towards enhanced pollutant as well as light absorption capability, which includes increased stability at various conditions and enhanced e–-h+ recombination hindrance. However, these studies involve toxic organic solvents which result in an environmentally unsafe condition and additional time-consuming multi-step processes that make the whole process more tedious and less economical.
Therefore, in order to overcome these drawbacks, synthesis of new nanomaterials with a suitable method to control the size, shape, and morphology would be an alternative for the existing materials. Different chemical synthesis techniques including the hydrothermal, precipitation, sol-gel, impregnation, micro-emulsion, solvothermal, electrochemical deposition, microwave, etc. have been tested.15,16 Relative to the others, the sol-gel, co-precipitation, and impregnation methods showed positive effects.17
The present work aims to synthesize TAOs nanomaterial by impregnating various weight percentages of AO in TiO2 (TO) using an organic solvent-free impregnation method at different calcination temperature. The synthesized TAOs were characterized by scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX), X-ray diffraction (XRD), Fourier transform infrared (FT-IR), ultraviolet-visible spectroscopy (UV-Vis). Finally, the evaluation of as-synthesized TAOs had been carried out for its adsorption-photocatalytic degradation activity for methyl orange (MO) dye.
Methods
The impregnation method was adopted as reported in authors earlier work inorder to load AO on TO powder.18 0.1 M of TAO materials were synthesized by adding the appropriate amount of TOpowder in an aqueous solution of aluminum nitrate nonahydrate ((Al(NO3)3·9H2O) salt with continuous stirring on a magnetic stirrer at 80oC for 30 minutes. The pH of the solution was adjusted by drop wise addition of NaOH. After aging for 8 hours, the samples were washed, dried overnight (105°C) and ground. Furthermore, to remove impurities and increase the crystallinity, samples were calcined at 500 °C. For all percentage of AO, the same procedure was followed to synthesize TAOs and samples were designated as TAO-4, TAO-8, and TAO-12 for the respective 4, 8, and 12% of AO. To check the effects of temperature, the percentage optimized catalyst (TAO-4) was calcined at 700 and 900°C.
The Langmuir experiment in the dark was conducted using a different initial concentration of MO and optimized 0.5 g of catalyst in 50 mL of Erlenmeyer flasks. The photocatalytic degradation experiments were conducted by contacting 0.5 g of catalyst powder with an aqueous solution of MO. Initially, to see the effect of adsorption without light irradiation, the reaction mixture was kept in the dark for 60 minutes with continuous stirring using magnetic stirrer. On the second step, after achieving adsorption-desorption equilibrium in the dark, the samples were irradiated. Throughout the photocatalytic reaction, 10 mL of MO solution was taken at defined intervals and the residual concentration was measured using a UV-Vis spectrophotometer at the wavelength of 465 nm.19
The concentration of MO left during the degradation process after time, t was estimated using equation 1:

The reaction rate constant (k) was estimated from the linear relationship of equation 2:

To test the favorability of the adsorption reaction in dark the linear plots of Langmuir were used.
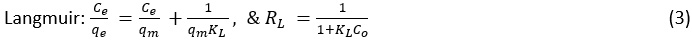
For the study of the spontaneity of the reaction process during adsorption test in the dark, the Flory-Huggins adsorption isotherm model was used.
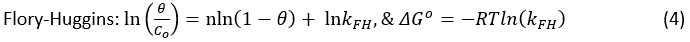
Where, Co is initial concentration; Ct is equilibrium concentration; qe is the amounts of analyte accumulated per gram of the catalyst; qm is maximum uptake; KL is the ratio of adsorption and desorption rate constant; n is the number of ions adsorbed on the site of the catalyst; = [1-(Ce/Co)] is fractional surface coverage; T is temperature (K); R is the universal gas constant (8.314 J/molK).
Results and Discussion
Characterization
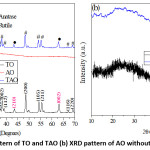
XRD patterns of TAO and amorphous AOare presented in Fig. 1a and b. In comparison with other results, the 2θ values and respective Miller indices for both bare TO and TAO appeared at 23o(101), 26o(110), 38o(004), 48o(200), 54o(105), 56o(211), 67o(110), and 69o(220) confirms the anatase TOand 35o(101), 44o(210), 63o(002) confirms the rutile TO.20,21 Even though the anatase phase was known for its excellent photodecomposition behavior, the presence of rutile phase in a mixture accounts for noticeable stability of the materials.14 The spectra for single TOand TAO appear to be identical except for a minor peak difference in width and height.
As shown in Fig. 2, at the calcination temperature of 500°C, the amorphous nature of AO is clearly observed before as well as after smoothing to 20 points. Furthermore, the particle size is also found to increase on rising the calcination temperature to 700 °C and 900 °C. As explained by,22 even though the formation of the gamma phase of aluminum oxides ( -Al2O3) was observed for single Al2O3 at higher calcination temperature (900 °C), the XRD patterns of TAO composite did not show the peaks for -Al2O3 (supplementary material, Fig. 1). Most probably, this is due to the domination of the higher percentage of TO in the composite. However, since the particle size is the key condition for photocatalytic degradation of dyes, TAO calcined at 500°C was used for the degradation of MO dye.
Figure 1: (a) XRD pattern of TO and TAO (b) XRD pattern of AO without and after smoothing
Figure 2: (a) SEM images at different magnification and (b) EDX spectra of TAO
SEM images and EDAX spectra of the synthesized materials were obtained from SEM-EDAX instrument with model ZEISS EVO 18 and are depicted in Fig. 2a. From SEM images, the presence of a spherical type of particles and a number of open pores between the particles were realized. These pores possibly enable the adsorption of the pollutant on the surface of the catalyst. Furthermore, the obtained EDAX spectra indicate the presence of titanium (Ti), aluminum (Al), carbon (C), and oxygen (O). The appearance of C peak is believed to be due to the standards used during analysis (Fig. 2a).
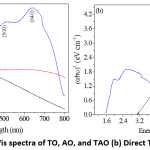
As illustrated in the UV-Vis spectra (Fig. 3), shifting of a peak to longer wavelength (redshift) from approximately 400 nm for TO to 500 and 640 nm for TAO, indicates the possibilities of degrading pollutants with visible light unlike that of TO which uses only UV light. The electronegativity values of Ti, Al, and O is 1.54, 1.61, and 3.4, respectively. The order of ionic nature of the elements is the inverse of their electronegativity. Therefore, the difference in energy levels between CB state of metal and VB of O 2P before contact attains the order of Ti >Al.23
Figure 3: (a) UV-Vis spectra of TO, AO, and TAO (b) Direct Taucs plot of TAO
Figure 4: FT-IR spectra of TO, AO, and TAO
This leads to the creation of a new bandgap in between valance band (VB) and conduction band (CB) of TO. It is reported, in fact, the amorphous nature of AO has more defect sites than crystals. By the synergistic effect, the e– – h+ recombination behavior decreases, thus increasing the photocatalytic activity.10 Therefore, this shifting of wavelength is believed to confirm the good impregnation of AO in the lattice of TO.
The FT-IR spectra (Fig. 4) of the synthesized materials shows no extra peaks for TAO compared to that of bare TO spectra. But, only a small change in width and intensity was observed. As usual, the peaks appeared approximately in the range of 400-900 cm-1 is possibly due to bending vibration of Ti-O, Al-O, Ti-O-Ti, and Al-O-Al. The other common broad stretching absorption bands detected at around 3400 and 1600 cm-1 are due to hydroxide ion (OH–) and water molecules (H-O-H). Those bands are important for adsorption of pollutant, especially during adsorption-desorption time.24,25
Photocatalysis
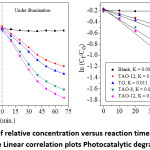
As shown in Fig. 5, TAO-4-500 exhibited the highest photocatalytic activity. This indicates that the percentage of AO affects the photocatalytic activity of the synthesized materials. As revealed on ln (Co/Ct) versus reaction time plot (Fig. 5b), except for the blank, all the other exhibited good linear correlation.
Figure 5: (a) Plots of relative concentration versus reaction time, (b) ln (Co/C) versus reaction time Linear correlation plots Photocatalytic degradation of MO
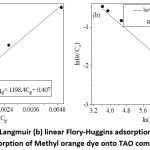
The order of the obtained equilibrium constant value follows the same trends as degradation efficiency order indicated above. Furthermore, as explained by Ohtani,26 to study the rate of reaction using first-order reaction kinetics in photocatalysis, the reaction should be under the control of the diffusion process. On the contrary, if the process is surface reaction limited, it should follow the Langmuir isotherm model. As shown in Fig. 6a, for this work the Langmuir model fits well and being the essential equilibrium parameter RL value in between 0 and 1 (i.e., 0.971) also indicates the actuality of favorable adsorption. Furthermore, the obtained negative free energy values ( = -2.74 kJ/mol) obtained from the Flory-Huggins model (Fig. 6b) show the spontaneity of the reaction process.
Figure 6: (a) Linear Langmuir (b) linear Flory-Huggins adsorption isotherm models for adsorption of Methyl orange dye onto TAO composite
Proposed possible Mechanisms
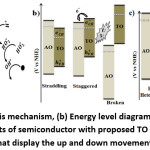
The band edges of metal oxide is highly dependent on the surface charge 27, therefore without conducting different analytical techniques it is difficult to determine the mechanism of formation of heterojunction between metal oxides. Therefore, the proposed possible mechanisms for this work were given in Fig. 7.
Figure 7: (a) Photocatalysis mechanism, (b) Energy level diagram signifying the three main electronic arrangements of semiconductor with proposed TO and AO mechanism, (c) Energy level diagram that display the up and down movement of the FL after contact
After the adsorption of pollutants on the active sites, as usual interaction of light (h ) with photocatalyst generates e– and h+ pair on CB and VB bands respectively. Moreover, interaction of these e– with oxygen from the air and h+ with water from the solution, led to the creation of a strong oxidizing agent, i.e. hydroxyl radicals ( ). Thus, the created leads to mineralization of pollutants to nontoxic CO2, H2O and mineral acids (Fig. 7a).
Depending on the bandgap energies and bandgap potentials of semiconductor metal oxide, relative to normal hydrogen electrode (NHE), the electronic arrangement of heterojunction categorized into straddling, staggered, and broke gap type (Fig. 7b). The charge transfer synergies of straddling and staggered gap are dependent on the amount of light energy applied and different synthesis condition. Due to great variation of the VB and CB location of two metal oxides, the charge transfer ability of broken type heterojunction is less applicable. Hence, among the three possible types heterojunction, the staggered gap type suggested as a precise coupling procedure. For the formation of the staggered gap type, it is reported that the up and down movement of Fermi level and forming contact to each other is the requirement (Fig. 7c). During this movement, a depletion layer that produces an electric field can be created. 28 The activated electric field drives the diffusion of the photo-generated e– – h+ out of the depletion layer and results in activation of the photocatalysis efficiency.29
Conclusions
Ti-Al Oxides (TAOs) nanomaterials were synthesized by impregnating various weight percentages of Al2O3 (AO) in TiO2 (TO) using an organic solvent-free impregnation method at different calcination temperatures. Among the three samples, the four percent AO calcined at 500 oC (TAO-4-500) exhibited a large surface area to volume ratio and better photocatalytic activity. All the XRD, SEM-EDX, FR-IR, and UV-Vis analytical techniques confirm the good impregnation of AO in TO lattice. The appearance of Ti, O, and Al surely indicates the purity of the materials and presence of AO in TO lattice. The obtained value of equilibrium constant for TAO-4-500 is 0.023. The evaluation of photocatalytic properties of TAOs on methyl orange (MO) revealed significant increase in efficiency with the decrease in the percentage of AO impregnated into TO lattice.
Acknowledgment
Authors are great full to the management of Adama Science and Technology University for providing financial support towards this work.
Funding Source
The paper is funded by Adama Science and Technology University.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article
References
- H. Guo, K. Lin, Z. Zheng, F. Xiao, and S. Li, Sulfanilic acid-modified P25 TiO2 nanoparticles with improved photocatalytic degradation on Congo red under visible light. Dyes and Pigments 92, 1278–1284 (2012).
- H. Deng, M. Zhang, Y. Cao, and Y. Lin, Decolorization of Reactive Black 5 by Mesoporous Al2O3 @TiO 2 Nanocomposites. Environ. Prog. Sustain. Energy 38, S230–S242 (2019).
- L. Wu, H. Yan, J. Xiao, X. Li, X. Wang, and T. Zhao, Characterization and photocatalytic properties of nano-Fe2O3–TiO2 composites prepared through the gaseous detonation method. Ceram. Int. 43, 14334–14339 (2017).
- D. Wang, Y. Wang, X. Li, Q. Luo, J. An, and J. Yue, Sunlight photocatalytic activity of polypyrrole – TiO2 nanocomposites prepared by ‘ in situ ’ method. Catal. Commun. 9, 1162–1166 (2008).
- S. Mianxin, B. Liang, Z. Tianliang, and Z. Xiaoyong, Surface ζ potential and photocatalytic activity of rare earths doped TiO 2. J. Rare Earths 26, 693–699 (2008).
- H. An et al., Deposition of Pt on the stable nanotubular TiO2 and its photocatalytic performance. Catal. Commun. 11, 175–179 (2009).
- X. Wang, S. Zhang, B. Peng, H. Wang, H. Yu, and F. Peng, Enhancing the photocatalytic efficiency of TiO2 nanotube arrays for H2 production by using non-noble metal cobalt as co-catalyst. Mater. Lett. 165, 37–40 (2016).
- S. MiarAlipour, D. Friedmann, J. Scott, and R. Amal, TiO2/porous adsorbents: Recent advances and novel applications. J. Hazard. Mater. 341, 404–423 (2018).
- H. Deng, Y. Wang, X. Zhang, X. Kou, B. Chen, and C. Zhu, Photodegradation under natural indoor weak light assisted adsorption of X-3B on TiO2/Al2O3 nanocomposite. Chem. Eng. J. 372, 99–106 (2019).
- A.A. Ismail, I. Abdelfattah, M.F. Atitar, L. Robben, H. Bouzid, S.A. Al-Sayari, D.W. Bahnemann, Photocatalytic degradation of imazapyr using mesoporous Al2O3–TiO2 nanocomposites. Sep. Purif. Technol. 145, 147–153 (2015).
- R. Ahmad, J. K. Kim, J. H. Kim, and J. Kim, Effect of polymer template on structure and membrane fouling of TiO2/Al2O3 composite membranes for wastewater treatment. J. Ind. Eng. Chem. 57, 55–63 (2018).
- M. Polat, A. M. Soylu, D. A. Erdogan, H. Erguven, E. I. Vovk, and E. Ozensoy, Influence of the sol–gel preparation method on the photocatalytic NO oxidation performance of TiO2/Al2O3 binary oxides. Catal. Today 241, 25–32 (2015).
- A. M. Soylu et al., TiO2–Al2O3 binary mixed oxide surfaces for photocatalytic NOx abatement. Appl. Surf. Sci. 318, 142–149 (2014).
- J.W. Lee, Y.I. Jang, W.S. Park, S.W. Kim, and B.J. Lee, Photocatalytic and Pozzolanic Properties of Nano-SiO2/Al2O3-TiO2 Powder for Functional Mortar. Mater. 12, 1037 (2019).
- F. Wang, X. Qin, Z. Guo, Y. Meng, L. Yang, and Y. Ming, Hydrothermal synthesis of dumbbell-shaped ZnO microstructures. Ceram. Int. 39, 8969–8973 (2013).
- Y. Fang, Z. Li, S. Xu, D. Han, and D. Lu, Optical properties and photocatalytic activities of spherical ZnO and flower-like ZnO structures synthesized by facile hydrothermal method. J. Alloys Compds 575, 359–363 (2013).
- A. Osman and H. Akbulut, A facile synthesis of zinc oxide / multiwalled carbon nanotube nanocomposite lithium ion battery anodes by sol e gel method Hilal K o. J. Power Sources 295, 235–245 (2015).
- B. Abebe and H. C. A. Murthy, Synthesis and Characterization of Ti-Fe Oxide Nanomaterials for Lead Removal. J. Nanomater. 2018, (2018).
- N. Davari, M. Farhadian, A. Reza, S. Nazar, and M. Homayoonfal, Degradation of diphenhydramine by the photocatalysts of ZnO/ Fe2O3 and TiO2/Fe2O3 based on clinoptilolite : Structural and operational comparison. J. Environ. Chem. Eng. 5, 5707–5720 (2017).
- Y. Wang, L. Li, X. Huang, Q. Li, and G. Li, New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 5, 34302–34313 (2015).
- A. S. Zhao, S. Zhou, Y. Wang, J. Chen, C. R. Ye, and N. Huang, Molecular interaction of fibrinogen with thermally modified titanium dioxide nanoparticles. RSC Adv. 4, 40428–40434 (2014).
- K. Atrak, A. Ramazani, and S. Taghavi Fardood, Green synthesis of amorphous and gamma aluminum oxide nanoparticles by tragacanth gel and comparison of their photocatalytic activity for the degradation of organic dyes. J. Mater. Sci.: Mater Electron 29, 8347–8353 (2018).
- R. Abdullah Mirzaie, F. Kamrani, A. Anaraki Firooz, and A. A. Khodadadi, Effect of α-Fe2O3 addition on the morphological, optical and decolorization properties of ZnO nanostructures. Mater. Chem. Phys. 133, 311–316 (2012).
- N. Abbas, G. N. Shao, M. S. Haider, S. M. Imran, S. Soo, and H. Taik, Sol – gel synthesis of TiO2-Fe2O3 systems : Effects of Fe2O3 content and their photocatalytic properties. J Ind. Eng. Chem. 39, 112–120 (2016).
- S W. Subramonian, T. Y. Wu, and S. Chai, Photocatalytic degradation of industrial pulp and paper mill ef fl uent using synthesized magnetic Fe2O3-TiO2 : Treatment ef fi ciency and characterizations of reused photocatalyst. J. Environ. Manage. 187, 298–310 (2017).
- B. Ohtani, Photocatalysis A to Z—What we know and what we do not know in a scientific sense. Photochem. Photobiol. C. Photochem. Rev. 11, 157–178 (2010).
- R. Beranek, (Photo) electrochemical Methods for the Determination of the Band Edge Positions of TiO2 -Based Nanomaterials. Advances in Physical Chemistry 2011, 1–20 (2011).
- A. Roy, Determination of the flatband potential of semiconductor particles in suspension by photovoltage measurement. Int. J. Hydrog. Energy 20, 627–630 (1995).
- M. Pirhashemi, A. Habibi-Yangjeh, and S. Rahim Pouran, Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 62, 1–25 (2018).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , and H C Ananda Murthy1
, and H C Ananda Murthy1

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal