Gulsum Aydin1 and Ayse Kalemtas2*
and Ayse Kalemtas2*
1Department of Biotechnology Selcuk University, Faculty of Natural Sciences, Konya, Turkey.
2Department of Metallurgical and Materials Engineering, Bursa Technical University, Faculty of Engineering and Natural Sciences, , Bursa, Turkey
Corresponding Author E-mail: ayse.kalemtas@btu.edu.tr
DOI : http://dx.doi.org/10.13005/msri/180107
Article Publishing History
Article Received on : 27-Nov-2020
Article Accepted on : 16-Mar-2021
Article Published : 20 Mar 2021
Plagiarism Check: Yes
Reviewed by: Dr. Kaushalendra Chaturvedi
Second Review by: Dr. Hussein Adel
Final Approval by: Dr. Bawadi Abdullah
Article Metrics
ABSTRACT:
Globally increased bivalve aquaculture production results in a vast amount of by-product discharges such as scallop shells. Utilization of these wastes to produce new products such as antibacterial agents can cooperate to reduce environmental problems and providea high value-added product at a lower cost. In this study, scallop shells are heat-treated at 800°, 900°, 1000°, and 1100°C for 4 hours at atmospheric conditions.X-ray diffraction analysis revealed that calcium carbonate is the only inorganic phase in the powdered scallop shells. After thermal treatment of the scallop shells, the calcium hydroxide phase was the only crystalline phase determined by X-ray diffraction analysis for the samples calcined at 1000° and 1100°C. At lower calcination temperatures, calcium carbonate and calcium hydroxide phases were co-existing in the samples. Scanning electron microscopy investigations depicted that using scallop shells as a starting material to synthesize nanometer-sized calcium hydroxide isachieved. It was determined that applied calcination temperature has a significant effect on the particle size of the obtained calcium hydroxide phase. Antimicrobial activity of calcined and uncalcined shell powders were tested against
Escherichia coli and
Staphylococcus aureus. No antibacterial activity was detected for the uncalcined scallop shell powders. However strong antibacterial activity was determined for the powders after subjection to calcination.Calcination of scallop shells is an environmentally friendly, readily applied, and low-cost approach to achieve nanometer-size calcium hydroxide that can be used as an inorganic antibacterial material in various composite systems.
KEYWORDS:
Antibacterial Activity; Calcination; Calcium Hydroxide; Scallop Shell;
Copy the following to cite this article:
Aydin G, Kalemtas A. Antibacterial Properties of Scallop Shell Derived Calcium Hydroxide Powders. Mat. Sci. Res. India; 18(1).
|
Copy the following to cite this URL:
Aydin G, Kalemtas A. Antibacterial Properties of Scallop Shell Derived Calcium Hydroxide Powders. Mat. Sci. Res. India; 18(1). Available from: https://bit.ly/3lyRRC1
|
Introduction
Bivalve aquaculture production, notably clam, oyster, mussel, and scallop culture, has been continuously increasing globally over the past few decades.1 Scallop shells are a by-product of the seafood industry that is cheap, abundant, and readily available. The annual discharge amount of the scallop shells is very high, and these wastes can cause environmental problems such asstrong unpleasant smell and soil pollution due to the heavy metal content of these shells. Scallop shells can be used to produce various food additives, paving materials,and plastering.2 Recently researches are focused on applications of scallop shells in various areas such asreinforcement for the polymer matrix composites 3, 4, desulfurization agent 5, filler in plywood adhesive 6, skin protective material7, 8,anti-obesity agent9,formaldehyde adsorbent 10, and adsorbent for radioactive substances such as Sr2+11.
Seafood industry wastes are valuable materials that can be used as a starting material to synthesize engineering materials that can be used in a wide variety of industrial applications.12-18 However, these materials are not utilized in most cases as a natural raw material source and are not recycledon a large scale to produce high-value-added industrial products. Recently intensive research is performed to utilize the scallop shells to achieve calcium-containing inorganic materials that can be used in various commercial applications.2, 19-23 Scallop shells are used to synthesize various inorganic materials such as calcium oxide (CaO)24, calcium hydroxide (Ca(OH)2), hydroxyapatite25, calcium titanate (CaTiO3)26, and aragonite (CaCO3)27-29. Recently, investigated applications of scallop shell derived calcium-containing materials are as a catalyst 19, 24, 30, 31, antibacterial reagent 2, 21-23, inactivating avian influenza virus32,limestone substitutes33, reinforcement for the composites 22, and sources to improve soil 20.
The scallop shells’ phase content is reported as 98–99 % calcium carbonate, trace inorganic materials, and 1–2 % organics.9It is well known that at elevated temperatures, organics are removed from the structure and calcium carbonate (CaCO3) inorganic phase converted to another phase,CaO. Achieved CaO has astrong antimicrobial activity, and it can also be used to produce various inorganic phases. Calcium hydroxide (Ca(OH)2) ceramic material is achieved via hydration of CaO, and it is known that Ca(OH)2 is a bactericidal material and is an effective candidate to be applied as a filler for the root canal in endodontic treatment applications.34 Both CaO and Ca(OH)2 and their composites with various materials can also be used as a catalyst for various reaction systems.35-40
In the current study, the aim was to synthesize an antibacterial inorganic material via heat treatment of waste scallop shells at four different temperatures (800°, 900°, 1000°, and 1100°C) at a constant dwell time, 4 hours. Characterization of the heat-treated scallop shells were carried out via X-ray diffraction and scanning electron microscopy investigations.The antimicrobial effects of the powdered as-received scallop shells and heat-treated scallop shellson gram-positive, and gram-negative bacteria were assessed through viable cell counts.
Experimental Studies
Reagents and Chemicals
Nutrient Broth (Merck) and agar powder (Himedia) were used for the microbiological tests. The bacterial isolates Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were a kind gift from Dr. Aslihan Kurt Kizildogan, 19 Mayis University, Samsun, Turkey. Distilled water was used throughout experiments. Scallop shells were collected from marine region ofMugla/Turkey. Collected shells were physically cleaned and washed with water to remove the impurities. Cleaned shells were rinsed with distilled water and dried in an oven overnight before the calcination process. Calcination studies were performed in air atmosphere at 4 different temperatures (800, 900,1000 and 1100°C) for 4 hours. Applied heating and cooling rate was 10°C/min. After calcination process samples were ground to the powder form in an agate mortar for the XRD analysis.Phase content of the samples were performed by X-ray diffraction (XRD, Bruker AXS/Discovery D8), using monochromatic Cu-Kα radiation (l=1.5406Å). Micro structure of the scallop shells and calcined scallop shell samples were investigated with a scanning electron microscope (SEM, Carl Zeiss/Gemini 300) in a secondary electron (SE) image mode.
Antimicrobial Activity Assay
The grounded as-received scallop shells and calcined scallop shells were sterilized at 121°C for 20 min prior to being used for antibacterial experiments. The bacterial strains, Escherichia coli (ATCC 25922) and Staphylococcus aureus(ATCC 25923)were grown in a nutrient broth for 18 h at 37°C. The cells were diluted to 107 CFU (colony forming units) per ml using nutrient broth and added into test tubes containing samples (0.2 %). The samples were incubated for 24 h at 37°C with agitation (200 rpm). At the end of the incubation period, samples were taken and diluted in saline and then grown on nutrient agar plates. Bacterial colonies were counted after 24 h of incubation at 37°C, and enumeration was performed in CFU/ml.
Results and Discussion
Macroscopic images of the as-received scallop shells before and after calcination at two different temperatures, 800° and 1100°C,are given in Fig. 1. Durig the calcination process organic content of the scallop shells are removed from the structure and a crystalline material is achieved as confirmed by the XRD analysis.
Figure 1: Macroscopic images of the scallop shells. (a) Before calcination and (b) after calcination at 800°C for 4 hours. (c) Before calcination and (d) after calcination at 1100°C for 4 hours.
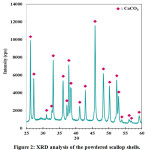
Phase analysis revealed that powdered as-received scallop shells contain only calcium carbonate phase (Fig. 2). This result is consistent with the literature reporting that scallop shells consist of calcium carbonate as an inorganic phase.41 Sawai et al.41 applied heat treatment to the scallop shells at 600°, 700°, 800°, 900°, and 1000°C for 1 hour. They reported XRD analysis results of the as-received and heat-treated scallop shells. Calcium carbonate was determined as an inorganic phase for the as-received and heat-treated scallop shells up to 700°C. 41 Sawai et al.41 reported that CaO was formed firstly at 800°C and at higher temperatures stronger CaO peaks were detected.
Figure 2: XRD analysis of the powdered scallop shells.
SEM investigations of the powdered as-received scallop shells are given in Fig. 3. Powdered scallop shells have a dense structure, and due to the applied milling procedure, fine particle size and relatively wide particle size distribution was achieved (Fig. 3). The powdered as-received scallop shells’ particle size is changing from a few micrometers size down to nanometer size.
Figure 3: SEM images of the powdered scallop shells at (a) 5000X and (b) 10000X.
Heat-treated scallop shells XRD analysis was performed approximately ten weeks after the heat treatment process. The heat-treated samples were stored at atmospheric conditions in a closed box. XRD analysis results revealed that the calcium hydroxide (Ca(OH)2) phase(portlandite, PDF 00-044-1481)was formed in all four calcined scallop shell samples. During the scallop shells’ heat treatment at >800°C, calcium carbonate (CaCO3) phase converted to CaOvia an endothermic reaction42 given below.
CaCO3(s)®CaO(s) + CO2(g)DH298K = 178 kJ/mol (Eq. 1)
Calcium carbonate has a very low hygroscopicity43 however, calcium oxide has a hygroscopic nature. It is known that when scallop shell-derived CaO is exposed to atmospheric conditions, Ca(OH)2 phase is formed.44 The hydration/dehydration reaction of CaO, also suitable for the thermochemical energy storage systems 45, is given in Eq. 2. Mihara et al.44 reported that three months after the heat treatment of the scallop shells, XRD analysis revealed that CaO reacted with the atmospheric moisture and Ca(OH)2 phasewas formed at the 900°C heat-treated scallop shell samples.
CaO(s) + H2O(g)«Ca(OH)2(s)DH298K = -104 kJ/mol (Eq. 2)
XRD analysis showed that the calcination temperature is a significant parameter on the phase content. When samples were calcined at 800 and 900°C calcium hydroxide (Ca(OH)2) and CaCO3 (vaterite, PDF 00-069-0001) phases were co-existing. At higher calcination temperatures vaterite phase was disappeared.
Figure 4: XRD analysis of the scallop shells calcined at (a) 800°C, (b) 900°C, (c) 1000°C, and (d) 1100°C for 4 hours.
Scanning electron microscopy investigations of the heat-treated scallop shells (Fig. 5)revealed that the particle size and distribution of the obtained calcium hydroxide phase slightly depends on the applied heat treatment temperature. Micro structural investigations revealed that the particle size and distribution of the heat-treated samples are very fine and homogenous for all samples. These nano meter sized calcium hydroxide powders can be used as a starting material to synthesize various ceramic materials such as calcium silicates and calcium phosphates.
Figure 5: SEM analysis of the scallop shells calcined at (a–b) 800°C, (c–d) 900°C, (e–f) 1000°C, and (g–h) 1100°C for 4 hours.
Effect of scallop shell powder and heat treated scallop shell powder on viability of E. coli and S. aureusis given in Table 1. The number of S. aureus and E. coli cells incubated with scallop shell powder for24h were 1.7×1010 CFU/ml and 3.1×109 CFU/ml respectively. On the other hand, number of S. aureus cells co-cultivated with shells calcined at 800, 900, 1000 and 1100°C were 1-5 CFU/ml. Growth of E. coli cells were totally inhibited that were co-cultivated with the shells calcined at 800-1100°C. Scallop shells calcined at all temperatures significantly inhibited growth of both the Gram negative E. coli and the Gram positive S. aureus compared to the number of viable cells incubated with the powdered as-received scallop shells. Oikava et al. 46 calcined shells of oyster, scallop,and clam and determined their antibacterial activities via total aerobic counts and E. coli. According to aerobic counts 4100 cells were determined in one millilitre of culture containing uncalcined scallop powder and 720 cells per milliliter were detected in the culture containing calcined scallop powder. The number of E. coli cells co-cultured with uncalcined scallop was 62 whereas no cells were determined in the culture containing calcined scallop. The authors have verified antibacterial activity of the calcined scallop shells by viable count which is in accordance with the results obtained in this study. Watanabe et al. 47 heated scallop shells and obtained nano particles,the main component of which was calcium hydroxide. Particles of different sizes were incubated with E. coli and the number of viable cells was counted to determine their antibacterial activity. The nano particles inhibited growth of E. coli in a dose dependent manner. Both Oikava et al.46 and Watanabe et al.47 reported the strong alkalinity of the aqueous solutions of the calcined powders to be responsible for the antibacterial activity. Calcium hydroxide dissociates into calcium and hydroxyl ions in an aqueous environment. The rapid release of hydroxyl ions resulting in a highly alkaline environment inhibits the growth of most microorganisms. Antimicrobial activity of calcium hydroxide is reported to be dependent on several mechanisms such as damage to the cytoplasmic membrane, inhibition of DNA replication and denaturation of proteins.48-50.
Table 1: Effect of grounded scallop shell and heat treated scallop shell powder son E. coli and S. aureus viability.
|
Samples
|
Number of viable cells (CFU/ml)
|
|
S. aureus
|
E. coli
|
|
SS
|
1.7×1010
|
3.1×109
|
|
SS–800
|
ND
|
ND
|
|
SS–900
|
ND
|
ND
|
|
SS–1000
|
ND
|
ND
|
|
SS–1100
|
ND
|
ND
|
SS: powdered scallop shell, SS–800: powdered scallop shell heat treated at 800°C, SS–900: powdered scallop shell heat treated at 900°C, SS–1000: powdered scallop shell heat treated at 1000°C, SS–1100: powdered scallop shell heat treated at 1100°C, ND: <5.
Conclusions
Using scallop shells as a starting material to synthesize nanometer-sized ceramic materials via calcination is an environmentally friendly, readily applied, and low-cost approach. XRD analysis showed that the calcination temperature is a significant parameter on the phase content. When samples were calcined at 800 and 900°C calcium hydroxide and vaterite phases wereco-existing. At higher calcination temperatures vaterite phase was disappeared.
Microstructural investigations revealed that the particle size and distribution of the heat-treated samples are very fine (nanometer size) and homogenous for all samples. SEM investigations revealed that the achieved calcium hydroxide particle size slightly depends on the applied calcination temperature.
Calcined scallop shells strongly inhibited the growth of E. coli and S. aureus. Calcium hydroxide dissociates into calcium and hydroxyl ions in an aqueous environment. The rapid release of hydroxyl ions resulting in a highly alkaline environment inhibits the growth of most microorganisms. Antimicrobial activity of calcium hydroxide is reported to be dependent on several mechanisms such as damage to the cytoplasmic membrane, inhibition of DNA replication and denaturation of proteins. 48-50
The results verified the potential of calcined shells as potential antimicrobial agents that can be used in various composite systems.
Acknowledgment
This project has been supported by the Foundation for Scientific Research Projects of Bursa Technical University (Project Number: 200 COVID 03).The authors wish to thank Seyda TAVSANOGLU (Mugla, Turkey) for supplying the scallop shells and Porland Porcelain (Bilecik, Turkey) for supplying the ceramic substrates. The authors gratefully acknowledge Bursa Technical University Central Research Laboratory (Bursa, Turkey) for the XRD and SEM analysis.
Conflict of interest
The authors declare that she/he has no conflict of interest regarding the publication of this article.
References
- D. Gallardi, Effects of bivalve aquaculture on the environment and their possible mitigation: a review, Fisheries and Aquaculture Journal 5(3) (2014).
CrossRef
- J. Sawai, Antimicrobial characteristics of heated scallop shell powder and its application, Biocontrol science 16(3) (2011) 95-102.
CrossRef
- B.S. Rajan, M. Balaji, M.A.N. AB, Effect of silane surface treatment on the physico-mechanical properties of shell powder reinforced epoxy modified phenolic friction composite, Materials Research Express 6(6) (2019) 065315.
CrossRef
- A.F. Hassan, R. Hrdina, Chitosan/nanohydroxyapatite composite based scallop shells as an efficient adsorbent for mercuric ions: Static and dynamic adsorption studies, International Journal of Biological Macromolecules 109 (2018) 507-516.
CrossRef
- H. Kim, G. Lu, T. Li, M. Sadakata, Binding and desulfurization characteristics of pulp black liquor in biocoalbriquettes, Environmental science & technology 36(7) (2002) 1607-1612.
CrossRef
- S. Yamanaka, K. Magara, Y. Hirabayashi, T. Fujimoto, Y. Kuga, Reduction of formaldehyde emission from plywood using composite resin composed of resorcinol–formaldehyde and urea-modified scallop shell nanoparticles, Wood Science and Technology 51(2) (2017) 297-308.
CrossRef
- Y.C. Liu, K. Uchiyama, N. Natsui, Y. Hasegawa, In vitro activities of the components from scallop shells, Fisheries science 68(6) (2002) 1330-1336.
CrossRef
- A. Torita, A. Miyamoto, Y. Hasegawa, The effects of scallop shell extract on collagen synthesis, Fisheries Science 73(6) (2007) 1388-1394.
- K. Takahashi, K. Satoh, M. Katagawa, A. Torita, Y. Hasegawa, Scallop shell extract inhibits 3T3-L1 preadipocyte differentiation, Fisheries science 78(4) (2012) 897-903.
CrossRef
- S. Yamanaka, A. Suzuma, T. Fujimoto, Y. Kuga, Production of scallop shell nanoparticles by mechanical grinding as a formaldehyde adsorbent, Journal of nanoparticle research 15(4) (2013) 1573.
CrossRef
- F. Mihara, Y. Shuseki, S. Tamura, K. Ui, K. Kikuchi, A. Yasumori, S. Komaba, M. Fukunishi, Y. Kogo, Y. Idemoto, Removal of strontium from aqueous solutions using scallop shell powder, Journal of the Ceramic Society of Japan 127(2) (2019) 111-116.
CrossRef
- P. Terzioğlu, H. Öğüt, A. Kalemtaş, Natural calcium phosphates from fish bones and their potential biomedical applications, Materials Science and Engineering: C 91 (2018) 899-911.
CrossRef
- I. Hamed, F. Özogul, J.M. Regenstein, Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review, Trends in food science & technology 48 (2016) 40-50.
CrossRef
- A. Hart, Mini-review of waste shell-derived materials’ applications, Waste Management & Research 38(5) (2020) 514-527.
CrossRef
- K. de la Caba, P. Guerrero, T.S. Trung, M. Cruz-Romero, J.P. Kerry, J. Fluhr, M. Maurer, F. Kruijssen, A. Albalat, S. Bunting, From seafood waste to active seafood packaging: An emerging opportunity of the circular economy, Journal of Cleaner Production 208 (2019) 86-98.
CrossRef
- V.P. Santos, N.S. Marques, P.C. Maia, M.A.B.d. Lima, L.d.O. Franco, G.M.d. Campos-Takaki, Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications, International journal of molecular sciences 21(12) (2020) 4290.
CrossRef
- G. Gecim, G. Aydin, T. Tavsanoglu, E. Erkoc, A. Kalemtas, Review on extraction of polyhydroxyalkanoates and astaxanthin from food and beverage processing wastewater, Journal of Water Process Engineering (2020) 101775.
CrossRef
- G. Aydin, P. Terzioğlu, H. Öğüt, A. Kalemtas, Production, characterization, and cytotoxicity of calcium phosphate ceramics derived from the bone of meagre fish, Argyrosomus regius, Journal of the Australian Ceramic Society (2020) 1-10.
CrossRef
- A. Buasri, N. Chaiyut, V. Loryuenyong, P. Worawanitchaphong, S. Trongyong, Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production, The Scientific World Journal 2013 (2013).
CrossRef
- S. Liang, J. Chen, J. Niu, X. Gong, D. Feng, Using recycled calcium sources to solidify sandy soil through microbial induced carbonate precipitation, Marine Georesources & Geotechnology 38(4) (2020) 393-399.
CrossRef
- M.S. Jeong, J.S. Park, S.H. Song, S.B. Jang, Characterization of antibacterial nanoparticles from the scallop, Ptinopecten yessoensis, Bioscience, biotechnology, and biochemistry 71(9) (2007) 2242-2247.
CrossRef
- C.-S. Wu, D.-Y. Wu, S.-S. Wang, Antibacterial Properties of Biobased Polyester Composites Achieved through Modification with a Thermally Treated Waste Scallop Shell, ACS Applied Bio Materials 2(5) (2019) 2262-2270.
CrossRef
- J. Sawai, H. Shiga, H. Kojima, Kinetic analysis of the bactericidal action of heated scallop-shell powder, International journal of food microbiology 71(2-3) (2001) 211-218.
CrossRef
- S. Kaewdang, R. Nirunsin, Synthesis of Calcium Oxide from River Snail Shell as a Catalyst in Production of Biodiesel, Applied Environmental Research 41(1) (2019) 31-37.
CrossRef
- Y.S. Firda, Y. Yusril, Influence of Ca/P concentration on hydroxyapatite (HAp) from asian moon scallop shell (amusium pleuronectes), (2019).
- I. Fatimah, R.N. Ilahi, R. Pratami, Low cost CaTiO 3 perovskite synthesized from scallop (Anadara granosa) shell as antibacterial ceramic material, IOP Conf Ser-Mat Sci, 2018, p. 012034.
CrossRef
- K. Sasaki, M. Hongo, M. Tsunekawa, Synthesis of aragonite-type of calcium carbonate from calcined scallop shell. 3rd Report. In aqueous system at ambient temperatures; Hai hotate kaigara shoseibutsu wo genryo to suru aragonite gata keishitsu tansan calcium no gosei. 3. Joon suiyoekikei ni okeru kojundo aragonite no seisei, (1998).
CrossRef
- K. Sasaki, M. Hongo, M. Tsunekawa, Synthesis of aragonite-type of calcium carbonate from calcined scallop shell. With amorphous calcium carbonate as an intermediate; Hai hotate kaigara shoseibutsu wo genryo to suru aragonite gata keishitsu tansan calcium no gosei. Hishoshitsu tansan calcium wo chukantai to suru hoho, (1997).
CrossRef
- S. Keiko, K. Hiroyuki, K.-K. Yoo, T. Masami, Morphologies of Aragonite Synthesized from Scallop Shells and Lime Stones by Successive Reaction, Proceedings of the IEEK Conference, The Institute of Electronics and Information Engineers, 2001, pp. 331-336.
- A. Buasri, P. Worawanitchaphong, S. Trongyong, V. Loryuenyong, Utilization of scallop waste shell for biodiesel production from palm oil–optimization using taguchi method, APCBEE procedia 8 (2014) 216-221.
CrossRef
- N. Chaihad, I. Kurnia, A. Yoshida, C. Watanabe, K. Tei, P. Reubroycharoen, Y. Kasai, A. Abudula, G. Guan, Catalytic pyrolysis of wasted fishing net over calcined scallop shells: Analytical Py-GC/MS study, Journal of Analytical and Applied Pyrolysis 146 (2020) 104750.
CrossRef
- C. Thammakarn, M. Tsujimura, K. Satoh, T. Hasegawa, M. Tamura, A. Kawamura, Y. Ishida, A. Suguro, H. Hakim, S. Ruenphet, Efficacy of scallop shell powders and slaked lime for inactivating avian influenza virus under harsh conditions, Archives of virology 160(10) (2015) 2577-2581.
CrossRef
- S. Her, T. Park, E. Zalnezhad, S. Bae, Synthesis and characterization of cement clinker using recycled pulverized oyster and scallop shell as limestone substitutes, Journal of Cleaner Production 278 (2020) 123987.
CrossRef
- L.C. Guerrero, J. Garza-Cervantes, D. Caballero-Hernández, R. González-López, S. Sepúlveda-Guzmán, E. Cantú-Cárdenas, Synthesis and characterization of calcium hydroxide obtained from agave bagasse and investigation of its antibacterial activity, Revista Internacional de Contaminación Ambiental 33(2) (2017) 347-353.
CrossRef
- A.M. Rabie, M. Shaban, M.R. Abukhadra, R. Hosny, S.A. Ahmed, N.A. Negm, Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil, Journal of Molecular Liquids 279 (2019) 224-231.
CrossRef
- C. Dang, L. Liu, G. Yang, W. Cai, J. Long, H. Yu, Mg-promoted Ni-CaO microsphere as bi-functional catalyst for hydrogen production from sorption-enhanced steam reforming of glycerol, Chemical Engineering Journal 383 (2020) 123204.
CrossRef
- B. Turkkul, O. Deliismail, E. Seker, Ethyl esters biodiesel production from Spirulina sp. and Nannochloropsis oculata microalgal lipids over alumina-calcium oxide catalyst, Renewable Energy 145 (2020) 1014-1019.
CrossRef
- T. Qu, S. Niu, Z. Gong, K. Han, Y. Wang, C. Lu, Wollastonite decorated with calcium oxide as heterogeneous transesterification catalyst for biodiesel production: Optimized by response surface methodology, Renewable Energy (2020).
- B. Fekhar, L. Gombor, N. Miskolczi, Pyrolysis of chlorine contaminated municipal plastic waste: In-situ upgrading of pyrolysis oils by Ni/ZSM-5, Ni/SAPO-11, red mud and Ca (OH) 2 containing catalysts, Journal of the Energy Institute 92(5) (2019) 1270-1283.
CrossRef
- J. Deng, H. Dong, L. Li, Y. Wang, Q. Ning, B. Wang, G. Zeng, Ca (OH) 2 coated nanoscale zero-valent iron as a persulfate activator for the degradation of sulfamethazine in aqueous solution, Separation and Purification Technology 227 (2019) 115731.
CrossRef
- J. Sawai, M. Satoh, M. Horikawa, H. Shiga, H. Kojima, Heated scallop-shell powder slurry treatment of shredded cabbage, Journal of food protection 64(10) (2001) 1579-1583.
CrossRef
- S. Lin, T. Kiga, Y. Wang, K. Nakayama, Energy analysis of CaCO3 calcination with CO2 capture, Energy Procedia 4 (2011) 356-361.
CrossRef
- R. Sullivan, M. Moore, M. Petters, S. Kreidenweis, G. Roberts, K. Prather, Effect of chemical mixing state on the hygroscopicity and cloud nucleation properties of calcium mineral dust particles, Atmospheric Chemistry and Physics 9(10) (2009) 3303-3316.
CrossRef
- Y. Mihara, T. Takada, N. Uno, I. Togashi, K. Sugimoto, Antibacterial and antifungal effects of fired scallop shell-glass composites fabricated by a simple process, J Ceram Soc Jpn Suppl 116 (2008) S1-S4.
CrossRef
- Y.A. Criado, M.n. Alonso, J.C. Abanades, Kinetics of the CaO/Ca (OH) 2 hydration/dehydration reaction for thermochemical energy storage applications, Industrial & Engineering Chemistry Research 53(32) (2014) 12594-12601.
CrossRef
- K. Oikawa, T. Asada, K. Yamamoto, H. Wakabayashi, M. Sasaki, M. Sato, J. Matsuda, Antibacterial activity of calcined shell calcium prepared from wild surf clam, Journal of Health Science 46(2) (2000) 98-103.
CrossRef
- T. Watanabe, R. Fujimoto, J. Sawai, M. Kikuchi, S. Yahata, S. Satoh, Antibacterial characteristics of heated scallop-shell nano-particles, Biocontrol science 19(2) (2014) 93-97.
CrossRef
- B. Athanassiadis, P. Abbott, L.J. Walsh, The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics, Australian dental journal 52 (2007) S64-S82.
CrossRef
- E. Schäfer, K. Bössmann, Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis, Journal of Endodontics 31(1) (2005) 53-56.
CrossRef
- B. Han, X. Wang, J. Liu, F. Liang, X. Qu, Z. Yang, X. Gao, The biological performance of calcium hydroxide–loaded microcapsules, Journal of endodontics 39(8) (2013) 1030-1034.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 and Ayse Kalemtas2*
and Ayse Kalemtas2*
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal