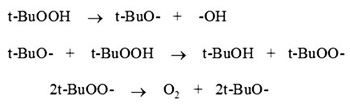Chemiluminescence Studies of Reaction Between Luminol and T-Butyl Hydroperoxide in Presence of Some Nsaids
S. A. Khan1, R. S. Kher2, A. L. S. Chandel3 and Priti Pandey3
1Government College, Seepat, Bilaspur, India.
2Department of Physics, Government ERR Science College, Bilaspur, India.
3Department of Chemistry Government ERR Science College, Bilaspur, India.
DOI : http://dx.doi.org/10.13005/msri/070136
Article Publishing History
Article Received on : 02 Feb 2010
Article Accepted on : 09 Mar 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The Chemiluminescence (CL) that accompanies the oxidation of luminol with tert-butyl hydroperoxide (t-BuOOH) in alkaline medium was studied. The reaction occurring between luminol and t-BuOOH in alkaline medium leads to the production of nitrogen gas with simultaneous emission of CL. The effect of non-steroidal anti-inflammatory drugs (NSAIDS) like aspirin and ibuprofen has also been studied. The time dependence of the CL intensity of luminol and t-BuOOH at different temperature and the CL emission spectra of the reactions have been recorded for better understanding of the reaction.
KEYWORDS:
Chemiluminescence; Luminol; T-Butyl hydroperoxide; Non-steroidal anti-inflammatory drugs like Aspirin & Ibuprofen.
Copy the following to cite this article:
Khan S. A, Kher R. S, Chandel A. L. S, Pandey P. Chemiluminescence Studies of Reaction Between Luminol and T-Butyl Hydroperoxide in Presence of Some Nsaids. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Khan S. A, Kher R. S, Chandel A. L. S, Pandey P. Chemiluminescence Studies of Reaction Between Luminol and T-Butyl Hydroperoxide in Presence of Some Nsaids. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2320
|
Introduction
Emission of energy in the form of visible light during chemical reaction is known as chemiluminescence (CL).1 CL has been found to be associated with variety of chemical reactions but in most of cases it has been observed due to redox reactions or free radical recombination.2 The reactions involving CL has been applied as an effective analytical tool to explore molecular phenomenon and has been found to be applicable in determination of many compounds.3-5 This method has also been employed as a diagnostic tool in immuno assay, receptor assay, DNA probes, bio sensors etc.6-7
It has been suggested that breakdown of organic hydroperoxide generates 1O2 (singlet oxygen). This singlet oxygen may be the source of the chemiluminescence (CL) observed during lipid peroxidation.8 Decompositon of organic hydroperoxides by metals could yield alcohols, ketones, peroxyl and alkyl radicals which are capable of generating CL. The decomposition of t-BuOOH catalyzed by transition metal ions or haeme compounds have been found to exhibit CL. The use of luminol increase the sensitivity of such CL reaction.9 In 1999, Tsaplev10 proposed an alternative light-producing pathway for the oxidation of linear hydrazides with hypochlorite, in which the formation of an electronically excited state of molecular nitrogen was discussed.
NSAIDS have been studied in term of their effect on the production of reactive oxygen species (ROS) since, in addition to having a widely accepted mechanism of action by inhibition of the enzymes involved in the production of pro-inflammatory lipid-derived mediators, these compounds may also interact with the oxidants produced by phagocytic cells.11-14 Antwerpen et al.15 studied the reactions of oxicam and sulfoanilide non steroidal anti-Inflammatory drugs with hypochlorous acid. In order to examine its reactivity with potential scavenging molecules from the non steroidal anti-inflammatory drugs family, a competition assay based on para-aminobenzoic acid (PABA) chlorination was developed. Earthwarmoorthy et al.16 studied chemiluminescence detection of paracetamol by a luminol-permanganate system
In view of the above it has been thought to explore the reaction of luminol and t-BuOOH in presence of nsaids to understand the mechanism of the reaction. Therefore present paper deals with chemiluminescence studies of reaction between luminal and t-butyl hydroperoxide in presence of some nsaids like aspirin & ibuprofen.
Experimental
The reagents used for present investigation were luminol, t-BuOOH , buffer solution having pH 13 and nsaids like aspirin and ibuprofen. All the chemical used in the present investigation were taken in solution form and the solutions were prepared by using AR grade material adopting standard method described in the textbook. An aqueous solution of all the chemical compounds were prepared in double distilled water. Solution of different concentration were prepared and tested. The concentration having most intense CL was determined and hence selected for further investigation.
Assembly for CL measurements essentially consisted of a chemiluminescent cell, high voltage power supply, light detector and a PC linked through interface. The kinetics of chemiluminescence was recorded with a RCA 931 photomultiplier tube (PMT); the photomultiplier was interfaced with a PC. The chemiluminescence cell and photomultiplier tube (PMT) were placed in a light box. Two circular holes made on the top surface of the box. One for placing syring to inject t-BuOOH in the reactor and other for placing thermocouple in the CL cell. The reactor is fitted inside the top surface of the light tight box and it rests just below the circular hole in which the syringe is placed. The reactors were highly transparent glass tube of 1.0 cm diameter and 5 cm length made by IMX machine (USA) situated in front of the entrance window of a photoreceiving device. The box was covered with black cloth and syringe was placed on the hole.
Results and Discussion
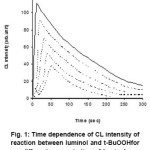
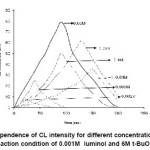
In order to study Chemiluminescence of luminol and t-BuOOH in presence of nsaids, we tried to detect CL by adding t-BuOOH in the aqueous solution of different concentration of luminol in a buffer solution having pH 13 as shown in figure 1. It has been observed that CL intensity increases with time, attains a maximum value, then decreases with time.
Figure 1: Time dependence of CL intensity of reaction between luminol and t-BuOOHfor different concentration of luminol.
During the present investigation it has been observed that the reaction of luminol and t-BuOOH shows CL, the time dependence of CL intensity of the reaction is shown in figure 1, which clearly indicates that the reaction leading to light emission is quite rapid. It was also observed that by increasing the concentration of luminol while keeping the concentration of peroxide constant, the CL intensity increases, which may be due to the greater production of light emitting species during the chemical reaction.
The probable mechanism explaning the above facts can be summarized as follows.
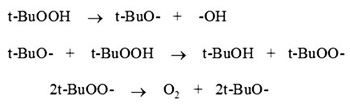
Scheme 1
The mechanism suggest that t-BuOOH decomposes to give O2 which reacts with luminol in alkaline medium to produce aminophthalate ion (excited state) which ultimately decomposes to singlet nitrogen, singlet nitrogen then produced luminescence.
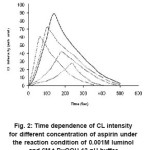
The reaction of luminol and t-BuOOH in presence of nsaids like aspirin and ibuprofen showed decrease in CL intensity which is indicated in the figure 2 and 3, showing large area under the CL intensity time curve with slower rise and decay kinetics. Therefore, the mechanism of CL emission is different in presence of nsaids compounds as compared to mechanism of luminol and t-BuOOH reaction. Many anti-inflammatory agent ( including some NSAIDS like, ibuprofen, aspirin etc ) act as scavenger of active oxygen species such as hydroxyl radical, superoxide anion radical etc.17-24
Figure 2: Time dependence of CL intensity for different concentration of aspirin under the reaction condition of 0.001M luminol and 6M t-BuOOH 13 pH buffer
Figure 3: Time dependence of CL intensity for different concentration of Ibuprofen under the reaction condition of 0.001M luminol and 6M t-BuOOH at 13 pH
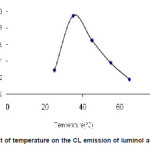
The temperature dependence CL intensity of luminol and t-BuOOH substantially increase as temperature grows attains an optimum value then further decrease with increase in temperature are shown in figure 4.
Figure 4: Effect of temperature on the CL emission of luminol and t-BuOOH
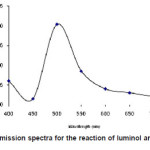
The chemiluminescence spectra of luminol and t-BuOOH system show a broad emission band with maximum emission at 485nm figure 5.
Figure 5: CL emission spectra for the reaction of luminol and t-BuOOH
The probable mechanism of the diminished CL intensity, effect of temperature and the CL spectra can be explained by suggesting following mechanistic pathway of the reaction.
t-BuOOH → t-BuO- + -OH
Aspirin + -OH → Aspirin – OH
(Aspirin spin adduct of ’”OH)
Similarly
Ibuprofen + ·OH → Ibuprofen – OH
(Ibuprofen spin adduct of ·OH )
Decrease in the concentration of ’”OH radical directly hampers the production of singlet oxygen which caused enhancement of CL intensity when it reacted with luminol as previously mentioned.
Conclusion
In summary, the CL behaviour of luminol with t-BuOOH & its subsequent decrease by nsaids (aspirin & ibuprofen) are reported. The proposed study undoubtedly could be applied for the detection of numerous analytes. It is hoped this study will
stimulate further investigation in this field.
Acknowledgments
The authors are thankful to Dr. A.K.Tripathi Principal, E. Raghvendra Rao Government Post Graduate Science College Bilaspur (C. G.) for providing experimental facilities and valuable suggestion.
References
- N. N. Ugarova, L. Yu. Brovoko, and E. L. Dementieva, Luminescence of solids Ed Vij D R Plenum press, New York (1998).
- Adam Waldemar, and Guiseppe Cilento, Chemical and Biochemical Generation of Excited states, Academic Press, NY (1982).
- Agatar, B. Irene, A.Roger, Jewsbury, Analytica Chimica Acta, 356: 247-254 (1998).
- Kumiko Saitoh, Takashi Hasebe, Norio Teshima, Makoto Kurihara, Tahuji Kawashima, Analytical Chimica Acta, 376: 247-254 (1998).
- Choiwan Lau, Jianzhong Lu; Massaki Kal, Analytical Chimica Acta 503: 235-239 (2004).
- H. H. Seiger, Photochemistry and photo biology, 21: 355-361 (1975).
- C. Dodeigne, L. Thunus, R. Lejeune, Talanta 51: 415-439 (2000).
- M. E. Murphy, H. Sies, Methods Enzymol, 105: 221 (1984).
- E. Cadenas, H. Sies, Methods Enzymol, 105: 221 (1984).
- V.R. Tsaples, Russ, J. Phys. Chem. 73: 1495 (1999).
- R. E. Kast, Biomedi-cal Pharmacotherapy, 54: 168-169 (2000).
- R. E. Kast, International Journals of Immuno pharmacology, 22: 1001-1006 (2000).
- C. Ju, And J. P. Uetrecht, Drug Metabolism and Disposition, 26: 676-680 (1998).
- J. W. G. Jacobs, and J. W. J. Bijlsma, Netherlands Journals of Medicine, 51: 198-204 (1997).
- P. V. Antwerpen, J. Dubois, M. Gelbcke, J. Neve, Free Radical Research, 38: 251-258 (2004).
- D. Easwaramoorthy, Y. C. Yu, and H. J. Huang, Analytica chemical acta, 439: 95-100 (2001).
- L. Pronai, Y. Ichilawa, K. Ichimori, H.Nakazawa, and S. Arimori, Journal of Clinical Biochemistry and Nutrition, 9: 17-23 (1990).
- R. Udassin, I. Ariel, Y. Haskel, N. Kitrossky, and M. Chevion, Free Radical Biology and Medicine,10: 1-6 (1991).
- A.K. Prakash, J.K. Sugden, Z. Yucan and W. Mingxin, International Journals of Pharmaceutics, 92: 151-155 (1993).
- K.O. Hiller, P.L. Hodd and R.L. Willson,Chemico-Biological Interactions,47: 293-305

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal