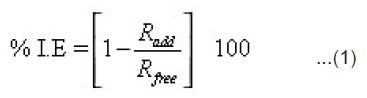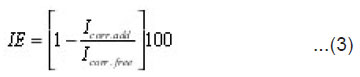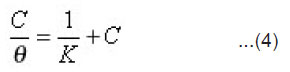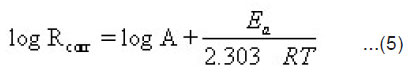Corrosion Inhibition of 1018 Carbon Steel with Some Ethoxylated Fatty Amides in Hydrochloric Acid Solution
F. M. Al-Nowaiser
Chemistry Department, King Abdulaziz University, P.O. BOX 80203 Jeddah, 2159, Saudi Arabia.
DOI : http://dx.doi.org/10.13005/msri/070115
Article Publishing History
Article Received on : 22 Mar 2010
Article Accepted on : 28 Apr 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The effect of three compounds of ethoxylated fatty amides with different number of ethylene oxide unit on the corrosion of 1018 carbon steel in 1M HCl has been studied using weight loss and galvanostatic polarization measurements. The percentage inhibition efficiency was found to increase with increasing concentration of inhibitor, number of ethylene oxide unit and with decreasing temperature. The inhibitive effect of these compounds was explained on the basis of adsorption on the metal surface, through their ethoxy groups. The adsorption process follows Langmuir adsorption isotherm. The effect of temperature on the rate of corrosion in the absence and presence of these compounds was also, studied. Some activated thermodynamic parameters were calculated.
KEYWORDS:
1018carbon steel; Ethoxylated fatty amide; Inhibitors
Copy the following to cite this article:
Al-Nowaiser F. M. Corrosion Inhibition of 1018 Carbon Steel with Some Ethoxylated Fatty Amides in Hydrochloric Acid Solution. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Al-Nowaiser F. M. Corrosion Inhibition of 1018 Carbon Steel with Some Ethoxylated Fatty Amides in Hydrochloric Acid Solution. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2249
|
Introduction
Acid solutions are generally used for the removal of undesirable scale and rust in several industrial processes. Hydrochloric acid is widely used in the pickling process of steel. The use of inhibitors is one of the most practical methods for protection against corrosion especially in acid solutions to prevent metal dissolution and acid consumption.1 Most well known acid inhibitors for steel corrosion are organic compounds containing hetero atoms.2-10 To be effective, an inhibitor must also displace water from the metal surface, interact with anodic or cathodic reaction sites to retard the oxidation and reduction corrosion reaction, and prevent transport ion of water and corrosion active species on the surface.11 Inhibitors which reduce corrosion on metallic materials can be divided into four kinds, (i) inorganic inhibitors, (ii) organic inhibitors, (iii) surfactant inhibitors and (iv) mixed materials inhibitors. Surfactants inhibitors have many advantages such as, for example, high inhibition efficiency, low price, low toxicity and easy production. Surfactants are molecules composed of a polar hydrophilic group, “the head”, attached to non polar hydrophobic group, “the tail”. In general, in aqueous solution the inhibitory action of surfactant molecules may also be due to physical (electrostatic) adsorption or chemisorption on metallic surface, depending on the charge of the solid surface and the free energy change of transferring a hydrocarbon chain from water to the solid surface.12-17 The adsorption of a surfactant markedly changes the corrosion resisting property of a metal, and for these reasons, studies of the relation between adsorption and corrosion inhibition are of considerable importance.18-20
The present investigations aim to study the effect of three ethoxylated fatty alcohols as corrosion inhibitors on the corrosion of 1018 carbon steel in 1M HCl using weight loss and galvanostatic polarization measurements. The effect of temperature on the dissolution of carbon steel in 1M HCl containing 1000 ppm of the inhibitors used was also studied and some thermodynamic parameters were computed.
Experimental
Carbon steel of type 1018 was used in this study has the chemical composition C 0.2%, Mn 0.6%, P 0.04 %, Si 0.003% and the remainder iron. Weight loss measurements were performed using compounds of the dimensions 3 x 3 x 0.1 cm3. For galvanostatic polarization polarization, a cylindrical rod embedded in araldite with exposed surface area of 0.2 cm2 was used. The electrodes were polished with different emery papers, degreased with acetone and rinsed by distilled water, before inserted in the test solution. For weight loss measurements, the steel coupons were left hanged in the test solution (1M HCl) for 150 minutes at 25 ± 1 °C before recording the loss of their weight.
The percentage inhibition efficiency (% I.E) and a parameter (q) which represents the part of the metal surface covered by the inhibitor molecules were calculated using the following equations.
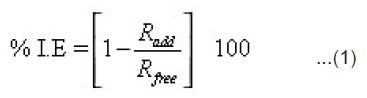

where Radd and Rfree are the corrosion rates of 1018 C-steel in free and inhibited acid solutions, respectively. The corrosion rate Rcorr (mg cm-2 min-1) was calculated from the slopes of the straight line obtained.
Galvanostatic polarization studies were carried out using a (PS remote) potentiostat with Zm PS6 software for calculation of corrosion parameters. Three compartment cell with a saturated calomel reference electrode was used. The inhibition efficiency IE was calculated using the following equation:
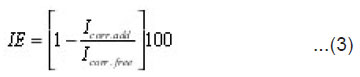
where Icorr.add and Icorr.free are the corrosion rates in free and inhibited acid, respectively.
The ethoxylated fatty amides which act also as non ionic surfactants were prepared using a simple method described elsewhere [15,21]. They have the general formula:
R-CONH-(CH2CH2O)n-H
H where R is C12H37 and n is the number of moles of ethylene oxide and equal 4,8,12 for compounds , I, II and III, respectively.
Results and Discussion
Weight Loss Measurements
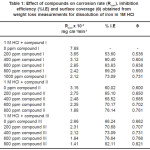
Fig. 1 shows the effect of increasing concentrations of compound III on the weight loss of 1018 carbon steel coupons vs time curves at 25 °C. Similar curves (not shown) were obtained for the other two compounds. It is obvious that the weight loss of carbon steel in presence of inhibitor varies linearly with time, and is much lower than that obtained in the blank solution. The linearlity obtained indicates the absence of insoluble surface film during corrosion and that the inhibitors were first adsorbed onto the metal surface and thereafter, impede the corrosion process.22 Values of IE and q obtained at different inhibitor concentrations are listed in Rcorr, Table 1. Inspection of Table 1 reveals that as the inhibitor concentration is increased, the weight loss decreases while IE and q increases. This behavior could be attributed to the increased surface coverage q due to the increase of the number of adsorbed molecules at the metal surface. At one and the same inhibitors concentration, the percentage of inhibition efficiency decreases in the following sequences.
compound III > compound II > compound I
Figure 1: Weight loss-time curves for the corrosion of carbon steel electrode in 1M HCl in absence and presence of different concentrations of compound III
Table 1: Effect of compounds on corrosion rate (Rcorr), inhibition efficiency (%I.E) and surface coverage (θ) obtained from weight loss measurements for dissolution of iron in 1M HCl
Adsorption Behavior
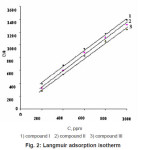
To study the adsorption behavior of ethoxylated fatty alcohols on 1018 carbon steel in 1M HCl, the adsorption isotherm must be defined. Attempts were made to fit θ values to various isotherms including Frumkin, Langmuir, Temkin and Freundlich isotherms. By far the results were best fitted by Langmuir adsorption isotherm. Langumuir adsorption isotherm could be represented using the following equation:
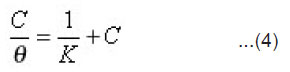
where K is the adsorption constant. Plotting Cθ against C gave a straight line with unit slope value (Fig. 2) indicating the adsorption of ethoxylated fatty alcohols on the steel surface follows Langmuir adsorption isotherm. From these results one can postulates that there is no interaction between the adsorbed species.
Figure 2: Langmuir adsorption isotherm
Effect of Temperature
The effect of temperature on the corrosion rate of 1018 carbon steel in free 1M HCl containing 1000 ppm of three compounds was tested in the temperature range of 30-60 °C using weight loss measurements. Similar curves to Fig. 1 were obtained (not shown).
As the temperature increases, the rate of corrosion increases and hence the inhibition efficiency of the additive decreases. This is due to the desorption is aided by increasing the temperature. This behavior proves that the adsorption of inhibitors on C-steel surface occurs through physical adsorption.
The activation energy (Ea) of the corrosion process was calculated using Arrehenius equation [23]
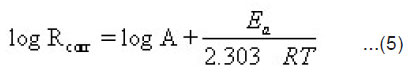
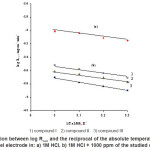
Fig. (3) represents Arrehenius plot (log Rcorr vs 1/T) for uninhibited and inhibited 1M HCl containing 1000 ppm of the studied compounds. The values of Ea can be obtained from the slope of the straight lines were found to be 16.253 KJ mol-1 in 1M HCl and 20.205, 22.043 and 24.655 kJ mol-1, respectively.
The increase of the activation energy in the presence of inhibitors is attributed to an appreciable decease in the adsorption process of the inhibitors on the metal surface with increase of temperature and a corresponding increase in the reaction rate because of the greater area of the metal that is exposed to the acid.24
Figure 3: Relation between log Rcorr and the reciprocal of the absolute temperature of 1018 carbon steel electrode in: a) 1M HCl, b) 1M HCl + 1000 ppm of the studied compound
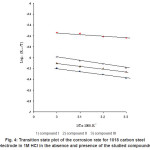
Figure 4: Transition state plot of the corrosion rate for 1018 carbon steel electrode in 1M HCl in the absence and presence of the studied compounds
The entropy of activation ( ΔS*) and the enthalpy of activation ( ΔH*) for dissolution of 1018 carbon steel in 1M HCl in presence of 1000 ppm of each used compound were obtained by applying the transition state equation.15
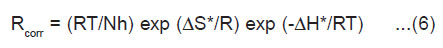
where N is Avogadro’s number, h is planck’s constant. A plot of log (Rcorr/T) vs (1/T) (Fig.4) should give straight line with a slope of (-ΔH*/2.303 R) and an intercept [log (R/Nh -Δ So / 2.303R)]. The values of DH* obtained from the slope of the straight line equal 10.253 KJ mol-1 in 1M HCl and equal 13.188, 17.213 and 19.220 KJ mol-1 in presence of compound I, II and III, respectively.
The values of ΔH* are different for studied compounds which mean that their structure affect the strength of its adsorption on the metal surface. The values of ΔS* calculated from the intercept of the straight line were found to 293.22 J mol-1 k-1 in 1M HCl and 308.15, 312.88 and 314.82 J mol-1 K-1 for compound I, II and III, respectively.
The negative values of ΔS* in the absence and presence of the inhibitors implies that the activated complex is the rate determining step and represents association rather than dissociation. It is also reveals that an increase in the order takes place in going from reactants to the activated complex.
Galvanostatic Polarization Studies
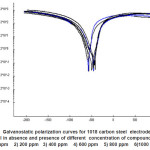
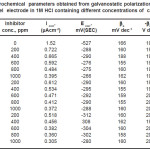
The effect of addition of ethoxylated fatty amides on the galvanostatic polarization curves of 1018 C-steel in 1M HCl solution was studied. The effect of increased concentration of compound III is represented in Fig.5 as an example. However, similar curves were obtained for the other compounds (not shown). The electrochemical parameters such as, corrosion current density (Icorr), corrosion potential (Ecorr.), anodic Tafel slope (βa), cathodic Tafel slope (βC), and inhibition efficiency (I.E.) were calculated and given in Table 2. Inspection of the data given in this table reveals that, as the concentration of the inhibitor increases, the values of ba and bc are approximately constant suggesting the inhibiting action of these compounds by adsorption at the metal surface according to blocking adsorption mechanism. The values of Ecorr. is shifted to more negative potentials, the values of Icorr. decreases and the values of IE increases indicating the inhibiting effect of these compounds. The inhibition efficiencies of these compounds decreases in the following order:
compound III > compound II > compound I
which is consistent with that obtained from weight loss measurements.
It is obvious that, the values of IE increased with an increase in the number of ethylene oxide unit. These finding could be explained on the bases of the fact that, an increase in concentration of the inhibitor would result in a lowering of the interfacial tension at the metal surface. This lowering in the interfacial tension is thought to a decrease of the bulk concentration of the inhibitor and an increase in its concentration at the metal surface.
Figure 5: Galvanostatic polarization curves for 1018 carbon steel electrode in 1M HCl in absence and presence of different concentration of compound III 1) 0 ppm 2) 200 ppm 3) 400 ppm 4) 600 ppm 5) 800 ppm 6)1000 ppm.
Table 2: Electrochemical parameters obtained from galvanostatic polarization technique of carbon steel electrode in 1M HCl containing different concentrations of carrageenas
Conclusions
The corrosion of 1018 carbon steel in 1M HCl is inhibited by ethoxylated fatty amides.
The inhibition efficiency was found to increase by increasing the inhibitor concentrations, number of ethylene oxide unit and/or decreasing the temperature.
The inhibitive effect of these compounds takes place through the adsorption of their compounds on the metal surface.
The adsorption process follows Langmuir adsorption isotherm.
The inhibition efficiency obtained from weight loss measurements showed good agreement with those obtained from comparative polarization measurements.
References
- J. M. Sykes: Br. Corros. J. 25: 175 (1990).
CrossRef
- M.Ozcan, I. Dehri and M.Erbil: Appl. Surf. Sci., 236: 155 (2004).
CrossRef
- K.F. Khaled : Appl. Surf. Sci., 230: 307 (2004).
CrossRef
- H.I. Wong, H. Fan, and J. Zheny: Mater. Chem.. & Phys., 77: 655 (2004).
CrossRef
- M.G. Hosseine, M. Ehteshamzadeh and T. Shahrabi : Electorchim. Acta, 25: 3680(2007).
CrossRef
- M. Abdallah, E.A.Helal and A.S. Fouda : Corros. Sci., 48: 1639 (2006).
CrossRef
- M. Abdallah; Material Chem & Phys. , 82: 786 (2003).
CrossRef
- M. Abdallah and M.M. El-Naggar; Mater. Chem. & Phys., 71: 291 (2001).
CrossRef
- I. Dehri and M. Ozcan: Mater. Chem & Phys., 98: 316 (2006).
CrossRef
- S. Ramesh and S. Rajeswari: Electrohimica Acta, 49: 81 (2004).
CrossRef
- A. Tizpar and Z. Ghasemi: Applied Surf. Sci., 252: 8630 (2006).
CrossRef
- M. M. Saleh: Mater. Chem. & Phys. 28: 83 (2006).
CrossRef
- L. Qiu, A. Xie and Yu. Shen: Corros. Sci., 47: 273 (2005).
CrossRef
- S.S. Abdel Rehim, H.H. Hassan and M.A. Amin: Appl. Surface Sci., 187: 279 (2002).
CrossRef
- M. Abdallah: Corros. Sci., 45: 2705 (2003).
CrossRef
- M.M. Osman: Mater. Chem. & Phys., 71: 12 (2001).
CrossRef
- M. Abdallah, A.A. El-Sarawy and A.Z. ElSonbati: Corros. Prev. & Control, 38(3): 97 (2001).
- M. Abdallah and A.Y. El-Etre: Portugatiae Electrochim. Acta. 21: 315 (2003).
CrossRef
- J. M. Bastidas, P. Pinilla, J. L. Polo and S. Miguel: Corros. Sci., 45: 427 (2003).
CrossRef
- M. Sahin, S. Bilgic and H. Yilmaz: Appl. Surf. Sci., 195: 1 (2002).
CrossRef
- E. Stipnisck-Lisac, A. Gazivoda and M. Madzarac: Electrochim. Acta, 47: 4189 (2002).
CrossRef
- F. Hanna, G.M. Sherbini and Y. Borakat: Br. Corros. J. 24: 269 (1985).
CrossRef
- I.N. Puitilova, S. Balezin, I.N. Barannik, V.P. Bishop: Metallic Corros. Inhibit., Pergamon, Oxford, 196 (1960).
- M. Abdallah: Corros. Sci., 46: 1981 (2004).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal