Corrosion Resistance of Tin Coated Implant Alloys in Artificial Physiological Solution
Kailash R. Jagdeo1*, Suresh N. Kadam2 and Methil R. Nair3
1Department of Physics, DSPM's K.V. Pendharkar College, Dombivli, India.
2Department of Physics, KET's V.G. Vaze College, Mulund, Mumbai, India.
3Model College, Dombivli, India.
DOI : http://dx.doi.org/10.13005/msri/070123
Article Publishing History
Article Received on : 20 Mar 2010
Article Accepted on : 28 Apr 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The corrosion behavior of TiN coated NiTi-Shape Memory Alloy, Ti6Al4V and 316L Stainless Steel implant alloys in artificial physiological solutions were investigated by means of electrochemical test. The cathodic arc physical vapor deposition method was used for film deposition. Surface morphology and surface composition were studied using SEM/EDAX and XRD. ICPAES was used to determine ions leached from each specimen when kept immersed in artificial physiological solution for sixteen weeks. The corrosion stability, elemental out-diffusion resistance, microhardness were found to be increased by this treatment.
KEYWORDS:
Corrosion; Shape memory alloys; Implant alloys; Cathodic arc physical vapor deposition
Copy the following to cite this article:
Jagdeo K. R, Kadam S. N, Nair M. R. Corrosion Resistance of Tin Coated Implant Alloys in Artificial Physiological Solution. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Jagdeo K. R, Kadam S. N, Nair M. R. Corrosion Resistance of Tin Coated Implant Alloys in Artificial Physiological Solution. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2274
|
Introduction
Nickel-Titanium (NiTi), shape memory alloy is useful in medical applications due to their super-elastic properties and shape memory effect.1-5, 22 NiTi is safe and bio-compatible in many in vitro and vivo studies due to its surface oxide film, which is mainly titanium oxide and it prevents from corrosion as well Ni leaching out from surface. The mechanical damage of superficial oxide film is noted to leach out nickel as it has high content of Ni.13 Nickel is noted to induce hypersensitive reactions and tissue necrosis. The induction of cancer by nickel ions is suspected.14 The toxicity of NiTi corrosion products has been noted in vitro15 and increase of Nickel in blood has been found after NiTi implanted into animals.16 Ti6Al4V alloy is also driving attention due to its excellent mechanical properties, corrosion resistance and super plasticity. Ti6Al4V alloy have a strong affinity for oxygen due to their high titanium content and promoting the formation of a stable and tightly adherent protective oxide layer on their surface. This is the reason for the good corrosion properties of this material. In biomedical applications, the chemical composition and stability of this surface oxide layer is very important because the surface of a biomaterial is in direct contact with biological tissues. Recently, some studies have shown that the possibility of vanadium release from Ti-6Al-4V in vitro and vivo, which is considered as a toxic element, may give rise to biocompatibility problems, altering the stability of this alloy and its viability as a biomaterial.17-21 It is also noted that NiTi and 316L stainless steel fall in higher grade in terms of anticorrosion.6 However, physicians frequently still have concerns against 316L stainless steel because of the contents Ni and Cr, which are both toxic. NiTi, Ti6Al4V and 316L stainless steel has proven safe and bio-compatible in many vitro and vivo studies but the leaching out of the harmful ions to the body tissues and fluids inside the human body has been raising much safety concerns. Their medical applications noted various medical complications such as allergic reactions, metallogenic and electrochemical reactions, metal toxicities and carcinogenicity.7-12 Therefore, proper passivity to prevent surface layer degradation and ions release into the environment is considered crucial for medical applications of implant alloys. In this study, cathodic arc physical vapor deposition technique is used for TiN coating to improve corrosion resistance of medical implant alloys. Samples are immersed in the plasma produced by the vacuum cathodic arc technique and negative bias is applied to them. The energetic ions having energy equal to neV (where n is the charge state of the ions and V is the applied bias voltage) are extracted from the plasma and are bombarded on the samples at nearly perpendicular direction. Nitrogen pressure during the deposition is maintained in the range of 10-3 torr. As the sheath of plasma takes the shape of the samples, samples having any complicated geometry can be uniformly bombarded by these ions.
Materials and Methods
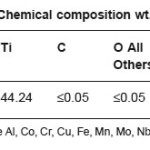
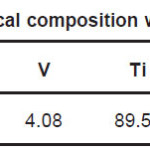
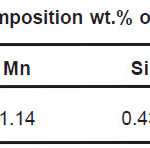
Samples in the sheet form of NiTi, Ti6Al4V and 316L stainless steel implant alloys were used in the present study. NiTi-shape memory alloy was procured from Johnson Metthey, USA. Chemical composition of NiTi, Ti6Al4V and 316L stainless steel implant alloys are shown in Table 1, Table 2, and Table 3 respectively.
Table 1: Chemical composition wt.% of NiTi
Table 2: Chemical composition wt.% of Ti6Al4V
Table 3: Chemical composition wt.% of 316L Stainless Steel
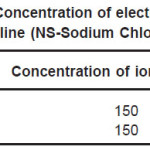
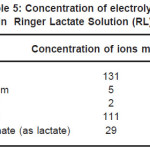
The sheets were cut into 12mm X 12mm square samples each having thickness 0.5mm. Samples were mechanically polished using SiC paper in successive grades from 200 to 2000 grit followed by a final mirror polish with a 0.5µm grade diamond-lapping compound. Samples were ultrasonically cleaned with acetone before coating. Cathodic arc physical vapor deposition technique was used to produce TiN coating on samples with process condition of bias voltage -200V, gas pressure 8 mtorr and deposition time 20 minutes. To get good adhesion of the deposited film with the samples, the samples were prior to deposition first sputter cleaned with Argon ions at pressure of 10 -4 torr and then heated to temperature of 400 0C. Increase in surface temperature helps in better bonding of film with the samples. This was done using radiant heater mounted in the chamber. The sample surface was also sputtered by titanium ion bombardment for three minutes. For this bombardment the titanium ions coming from arc source were accelerated towards the sample surface with the bias voltage of -1000V. Because of this Ti ions clean the substrate as well as they get implanted in the sample surface. The projected length of these ions is of the order of few nm. These implanted ions help in the formation of good adherent film. Six Ti cathodes with purity 99.95% were used. All coated samples were ultrasonically cleaned again before electrochemical test. Corrosion resistance of implant alloys were investigated by Tafel Extrapolation method. The electrochemical tests were carried out at room temperature, in Normal saline solution and Ringer Lactate solution. The concentration of electrolytes in Normal saline solution and Ringer Lactate solution is shown in Table 4 and Table 5 respectively.
Table 4: Concentration of electrolytes in Normal Saline (NS-Sodium Chloride 0.9%)
Table 5: Concentration of electrolytes in Ringer Lactate Solution (RL)
In dissolution test, six samples of surface area 1 cm2 of each type coated and bare were immersed in 50ml of Ringer Lactate solution in polypropylene bottles. The bottles were evacuated and closed tightly and incubated in thermostatic chamber at 37±10C for sixteen weeks. All bottles were shaken and rotated with a speed of 72 rpm. After 4, 8 and 16 weeks the solutions were analyzed by Inductive Coupled Plasma Atomic Emission Spectroscopy (ICPAES) to determine amount of Ni from NiTi, Al and V from Ti6Al4V and Cr from 316L stainless steel, leached out from samples. Microhardness measurements were performed on six different places of a sample by means of a microhardness tester of indenter type Vickers, duration time 5 seconds and test load of 10 g. Film thickness was determine by optical microscope examination of cross section through the coating. Thickness of TiN coating on all samples was found to be 1.5µm. Surface morphology and surface composition were studied using SEM/EDAX and XRD.
Results and Discussion
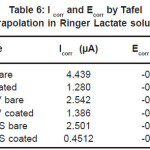
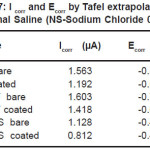
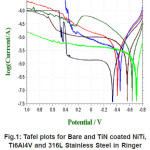
The essential readings acquired from the electrochemical tests are summarized in Table 6 and Table 7. The Tafel plots for alloys have been presented in Fig. 1 and Fig. 2.
Table 6: I corr and Ecorr by Tafel extrapolation in Ringer Lactate solution
Table 7: I corr and Ecorr by Tafel extrapolation in Normal Saline (NS-Sodium Chloride 0.9%)
Figure 1: Tafel plots for Bare and TiN coated NiTi, Ti6Al4V and 316L Stainless Steel in Ringer Lactate Solution at room temperature
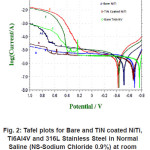
Figure 2: Tafel plots for Bare and TiN coated NiTi, Ti6Al4V and 316L Stainless Steel in Normal Saline (NS-Sodium Chloride 0.9%) at room temperature
Corrosion of bare NiTi, Ti6Al4V and 316LSS alloys in Ringer Lactate solution shows the order of corrosion resistance 316LSS>>Ti6Al4V>>NiTi. For TiN coated NiTi, Ti6Al4V and 316LSS the order of corrosion resistance is 316LSS>>NiTi>>Ti6Al4V. Reduction in the value of Icorr from bare to coated due to TiN coating shows the order NiTi>>316LSS>>Ti6Al4V.
TiN coating is best coating technique for reduction in value of Icorr from bare to coated for NiTi rather than for 316LSS and Ti6Al4V in Ringer Lactate solution. TiN coating shows improvement in corrosion resistance for NiTi, Ti6Al4V and 316LSS in Ringer Lactate solution. TiN coated NiTi shows much better improvement in corrosion resistance than TiN coated Ti6Al4V alloy in Ringer Lactate solution.
Corrosion resistance of bare NiTi and Ti6Al4V is found to be comparable but lower than that of 316L stainless steel in Normal Saline solution. The order of corrosion resistance is 316LSS>> NiTi>>Ti6Al4V. For TiN coated NiTi, Ti6Al4V and 316LSS the order of corrosion resistance is 316LSS>>NiTi>>Ti6Al4V. Reduction in Icorr value due to TiN coating, the order is 316LSS>>Ti6Al4V>> NiTi>>. TiN coating is best coating technique for reduction in Icorr for 316LSS rather than for NiTi and Ti6Al4V in Normal Saline solution. TiN coating shows improvement in corrosion resistance for NiTi, Ti6Al4V and 316LSS in Normal Saline.
The Ringer Lactate solution is more corrosive medium than Normal Saline solution for all alloys considered here for study. Bare NiTi shows good corrosion resistance in Normal Saline solution than Ringer Lactate solution. But TiN coated NiTi shows almost equal Icorr value in both electrolyte solutions.
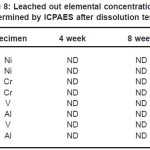
The dissolution of Ni from NiTi, Al and V from Ti6Al4V and Cr from 316L stainless steel from bare and TiN coated samples is shown in Table 8. The Cr and V were not detected (ND) even after 16 weeks dissolution test of bare and coated 316L stainless steel to the limit of 50 ppb. Al was detected but coating reduces the leaching out from surface by one third of its bare samples in sixteenth week for Ti6Al4V alloy. For bare NiTi, Ni leached out from surface was detected in sixteenth week and found to be 60ppb. But for TiN coated NiTi it was not detected.
Table 8: Leached out elemental concentrations determined by ICPAES after dissolution test
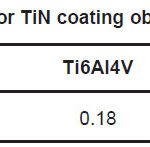
Wt.% ratio N/Ti of TiN film prepared under the deposition conditions, nitrogen gas pressure of 8 mtorr, bias voltage -200V and deposition time 20 minutes is shown in Table 9. The presence of small amount of oxygen in the film was noted. This could be due to contamination of film while handling in open atmosphere before EDAX. Equal wt.% of Nitrogen and Titanium in the film stoichiometry was observed in 316L stainless steel. In case of NiTi and Ti6Al4V alloy wt.% of Titanium is more than Nitrogen in film composition.
Table 9: N/Ti wt. % ratio for TiN coating obtained from EDAX study
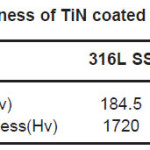
Table 10: Microhardness of TiN coated and bare samples
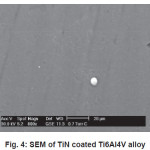
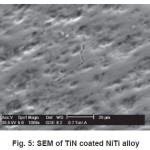
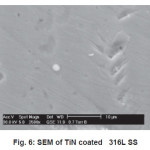
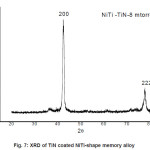
SEM micrograph of samples deposited at -200V bias voltage is shown in Fig. 3 to Fig 6. SEM micrograph of TiN coated implant alloys show droplets like defects. This effect can cause the observed corrosion behavior. It is also noted under best deposition condition, a superior corrosion resistance was observed for coating prepared by cathodic arc physical vapor deposition and reduces the droplet like defects. Fig 7 shows X-ray diffraction pattern for TiN coated NiTi-shape memory alloy. X-ray diffraction pattern for TiN coated Ti6Al4V and 316L stainless steel are not presented here. All X-ray diffraction pattern shows strong (200) preferred orientations.
Figure 3: SEM of TiN coated Ti6Al4V alloy
Figure 4: SEM of TiN coated Ti6Al4V alloy
Figure 5: SEM of TiN coated NiTi alloy
Figure 6: SEM of TiN coated 316L SS
Figure 7: XRD of TiN coated NiTi-shape memory alloy
Conclusion
Corrosion resistance of bare NiTi, Ti6Al4V alloys is found to be comparable but lower than that of 316L stainless steel in Normal Saline solution. Corrosion of bare NiTi, Ti6Al4V and 316L SS alloys in Ringer Lactate solution shows the order of corrosion resistance 316L SS>>Ti6Al4V>>NiTi. TiN coating shows improvement in corrosion resistance for NiTi, Ti6Al4V and 316L SS in both Normal Saline and Ringer Lactate solution. Microhardness of TiN coated 316L SS is more than TiN coated Ti6Al4V and NiTi alloys. SEM micrograph of TiN coated implant alloy shows droplets like defects. X-ray diffraction pattern for TiN coated implant alloys shows strong (200) preferred orientations.
Acknowledgements
The authors would like to thanks Prof. A. S. Khanna, IIT, Mumbai; Prof. D. C. Kothari, University of Mumbai, Mumbai; Ashok Jani, Multi-Arc India, for their technical and laboratory assistance. Financial assistance, Mumbai University Minor Research Project-(Physics-86, 2008-09).
References
- J. Ryhanen “Biocompatibility evaluation of nickel-titanium shape memory alloy”, Ph.D thesis, University of Oulu, Finland, (1999).
- Svetlana A. Shabalovskaya, Biomed. Materials and Eng. 12: 69 (2002)
- T. duerig, A Peltron, D. Stockes, Mater. Sc.Eng. 149: 273 (1999)
- L. El Medawar P Rocher, J. C. Hornez, M.Trasnel, J. Brame, H. F. Hildebrand, Biomed.Eng. 19: 153 (2002).
- Jinfang, Guo etal, “The proceeding on the international symposium on shape memory alloys”, Guilin, China, The Nonferrous Metals Society of China, China Acad. Publ. 379(1986).
- R. S. Dutta, Madangopal K., H. S. Gadiyar and S. Banergee “ Corrosion behavior of Ni-Ti shape memory alloy”-Metallic corrosionprincipals and control-Pbl.-Wiley Easternal Ltd.-New age international Ltd.- 112 (1994)
- M. Long and H. J. Rack, Biomaterials 19:1621 (1998)
CrossRef
- S. G. Steinemann, S. M. Parren, Titanium Science Technolgy, 2: 1327 (1985).
- W. Wiltshire, M. Ferreira and J. Lightelm, Quintessence Int. 27: 513 (1996)
- S. Lacy, K Meritt, S. Browm and A. Puryear, J. Biomed. Mater. Res. 32: 279 (1996)
CrossRef
- R. Hayes, Cancer causes and Control, 8: 321(1997)
CrossRef
- A. Oller and M. Costa, Toxicol. App. Pharmac.143: 152 (1997)
CrossRef
- C.C. Shih, S. J. Lin, K. H. Chung, Y. L. Chen,Y. Y. Su, J. Biomed. Mater. Res. 52: 323(2000).
CrossRef
- J. C. Wataha, N. L. O’ Dell, B. B Singh, M.Ghazi, G. M. Whitford, P. E. Lockwood, J.Biomed. Mater. Res. 58: 537(2001).
CrossRef
- C.C. Shih, S. J. Lin, Y. L. Chen, Y. Y. Su, S. T. Lai, G. J. Wu, C. F. Kwok, K. H. Chung, J.Biomed. Mater. Res. 52: 395 (2000).
CrossRef
- M. Assad, A. V. Chernyshov, P. Jarzem, M.A. Leroux, C. Coilard, S. Charette, C. H.Rivard, J. Biomed. Mater. Res. 64: 121(2003).
CrossRef
- Wapner K.L., Clin Orthop. 271: 12 (1991)
- Castaneda D.F., Evaluation of Gamma-TiAl as an Implant Material. M.S Thesis University of Puerto Rico, Mayaguez (2003)
- Garcia-Alonso M.C., Saldana L., Valles G.,Gonzalez-Carrasco J.L., Gonzalez-CabreroH., Martinez M.E., Gil-Garay E., Munuera L., Biomaterials 24: 19 (2003)
- Solar, R.I., Pollack, S.R., Korostoff E.,J.Biomed. Mater. Res. 13: 217 (1979).
CrossRef
- Milosev I., Metikos-Hukovic M., Strehblow H.,Biomaterials 21: 2103 (2000).
CrossRef
- R. Dutta, K. Madangopal, H. Gadiyar and S. Banerjee, Brit. Corr. J. 28: 217 (1993).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal