Corrosion Studies of Al-Cu Particulate Composites Produced Through Liquid Metallurgy Route
S. Madhusudan1*, M.M.M. Sarcar2, N.R.M.R. Bhargava3, K.V. Rao4
1Mechanical Engineering Department, Sir C.R.Reddy College of Engg, Eluru - 534 007, India.
2Mechanical Engineering. Department, A.U College of Engineering, Visakhapatnam - 530 003, India.
3Metallurgical Engineering. Department, A.U College of Engineering, Visakhapatnam - 530 003, India.
4Mechanical Engineering Department, Sir C.R.Reddy College of Engineering, Eluru - 534 007, India.
DOI : http://dx.doi.org/10.13005/msri/070142
Article Publishing History
Article Received on : 10 Dec 2009
Article Accepted on : 10 Jan 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Attempts have been made to disperse fine Cu filings in aluminium matrix by conventional stir cast technique to produce metal-metal composites. SEM-EDX analysis shows a gradual variation in chemistry from dispersoid to the matrix. Production of composites show an ease of dispersion of metallic dispersoid compared to a ceramic one. Corrosion study in terms of loss in weight in acid media has been investigated. Composite show a lesser dissolution rate compared to the alloy having same composition, at different concentrations of the corrosive media. Composite with higher concentration of dispersoid show reduced corrosion resistance.
KEYWORDS:
Stir casting; Dispersion; Metal-metal Composite; Microstructure; Corrosion
Copy the following to cite this article:
Sarcar M. M. M, Bhargava N. R. M. R, Rao K. V, Madhusudan S. Corrosion Studies of Al-Cu Particulate Composites Produced Through Liquid Metallurgy Route. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Sarcar M. M. M, Bhargava N. R. M. R, Rao K. V, Madhusudan S. Corrosion Studies of Al-Cu Particulate Composites Produced Through Liquid Metallurgy Route. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2339
|
Introduction
Metal matrix composites (MMCs) with ceramic reinforcement are known for their high specific strength, modulus and hardness properties. They share a good fraction in transport and aerospace applications. Fabrication, secondary processing, characterization are still pose a major hurdle in the manufacturing of these composites. Present investigation aims in fabrication of composite material which are in tune with the conventional alloys by dispersing solute metal as particulates, which posses the useful properties of the metal, alloy and the resultant composite. Comparative studies are made among alloy and composites on weight loss basis at different concentrations of corrosive media through rapid corrosion test.
Material and Methods
Fabrication of Alloy and Composites
Al-5 wt% Cu alloy is prepared by adding EC grade copper in the form of chunks to the IE grade molten aluminum.1 Copper powders are produced by filing a rotating copper rod at high speeds with different grades of files. Powders in different sizes are produced by different rotation speeds and files. Powders are classified by sieving methods. Composite metals are prepared by stir cast technique, adding preheated (2000C) copper powders to the molten aluminium at 7200C.2 Composite metals with 5, 10 and 15% Cu, by weight, are produced with combination of particle sizes-50+100 (0.225 microns). Both alloy and composites are cast into fingers of 150X 18mmÖ. Castings are homogenized at 1000C for 24 h.
Corrosion Test
Standard samples of 18mmÖ X 10mm have been prepared for conducting the test. All the samples were polished to have a uniform smoothness. The samples are thoroughly degreased using mild soap and washed with distilled water. The samples are dried at 1000C for 1 hr and placed in desiccator. Standard solution corrosive media of 1 liter capacity with 5% HCl and 95% water have been prepared. Equal volumes of 250 ml placed in 4 beakers of 500 ml capacity samples are placed in category wise (alloy, 5%, 10%, 15% composites) in the solution. Care has been taken to keep at least 1 inch difference for each sample. Samples were taken out at an interval of 2hr and thoroughly washed with distilled water. Dried samples were dipped in alcohol and weight loss is measured after drying.
Results and Discussions
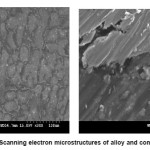
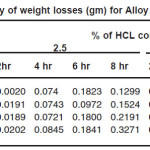
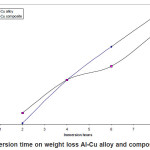
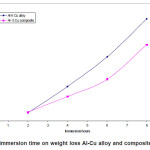
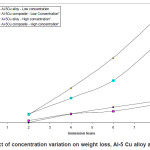
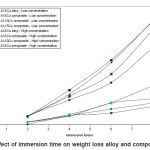
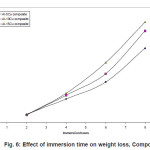
Figure.1 (a) reveals a well defined dendritic structure while (b) shows the copper particle in the aluminium matrix. SEM-EDX analysis shows a gradual variation in chemistry from dispersoid to the matrix3. Table 1 shows the summary of weight losses with varying percentages of corrosive media and different immersion times. Figure 2 shows the effect of immersion time on weight loss in solution having 2.5% HCl. Both alloy and the composite with same concentration of Cu, show a similar trend of dissolution, i.e, a gradual increase in weight loss with time. However, composite show a better resistance towards dissolution than the alloy. In case of alloy, presence of CuAl2 makes it susceptible to corrosion action in the presence of Cl-ions.4 Similar effect is also expected with the composite, but to a very little extent, as the alloy formation takes to a restricted region, i.e., where the Cu particles interacts with the matrix, resulting a dilute alloy in the vicinity of it. Presence of fine Al2O3 layer on the overall composite reduces this effect further, resulting in reduced dissolution. Further increase in Cu contents with the composites has shown increased dissolution amounts, figure 3. Increase in Cu content enhances the alloy formation, which in turn increases the fraction of CuAl2. As the CuAl2 increases, susceptibility to corrosion action enhances.4 On further increase in Cu contents (Al-15Cu composite), agglomeration of particles results in formation of Al-Cu rich alloys locally, which in turn enhances localized corrosion. The same can be seen with increased rates of dissolution, figure 4. Figure 5 depicts the dissolution behaviour of the alloy and the composite having the same composition at different solution concentrations. The severity of corrosion increases with increase in chloride concentration.4 Both the alloy and the composite respond in a similar fashion. At higher concentration, the severity of the corrosive attack is more severe with the alloy than the composite having the same composition. Localized corrosion occurs between the intermetallic particles, matrix and the interface between them. The earlier discussion holds for this kind of behaviour as the corrosive attack on CuAl2 in alloy is more severe at higher concentrations than passive Al2O3 layer on composite. Both alloy and the composite exhibit a similar behaviour at higher concentration of chloride ions, figure 6.
Figure 1: Scanning electron microstructures of alloy and composite
Table 1: Summary of weight losses (gm) for Alloy and Composites
Figure 2: Effect of immersion time on weight loss Al-Cu alloy and composite (2.5% HCl conc.)
Figure 3: Effect of immersion time on weight loss Al-Cu alloy and composite (5% HCl conc.)
Figure 4: Effect of concentration variation on weight loss, Al-5 Cu alloy and composite
Figure 5: Effect of immersion time on weight loss alloy and composites
Figure 6: Effect of immersion time on weight loss, Composites
Conclusions
Metal-metal composites can be produced through conventional foundry routes.
Composites with lower concentration of metallic reinforcements exhibit better corrosion resistance than the alloy of same concentration.
Increased metallic reinforcement concentration decrease corrosion resistance of the resultant composite.
Acknowledgements
It is great pleasure to express my gratitude and indebtedness to my supervisors Dr. MMM. Sarcar and Dr.NRMR.Bhargava for their guidance, encouragement, moral support and affection through the course of my work. Thanks are due to Dr.J.Babu Rao (AU,VSP), Dr.R.N.Rao (NIT, Warangal), Dr. Kamalluddin (Principal, Chirala Engg college, A.P), Dr. J.Appa Rao (Guntur), Sri Ch.Raminaidu (singapore) and Sri.K.Venkateswara Rao (HOD Mech, Sir CRR college of Engineering, Eluru ) for their support and timely help during my experimental work. I am also greatful to the authorities of Sir CRR College of Engineering, Eluru for their whole hearted support. Last but not least I would like express my sincere thanks to all my colleagues for their direct and indirect help.
References
- U Ravi Kiran, A Venugopala Naidu, J Babu Rao and NRMR Bhargava–Metal-metal composites: An innovative way for multiple strengthening–Special Materials Processing & Characterization, RRL, Bhubaneswar, India, (2001).
- NRMR Bhargava, I Samajdar, S Ranganathan and MK Surappa-Role of cold work and SiC reinforcements on the â’/â precipitation in Al-10Mg alloy, Met. Mat. Trans A. 29A (1998).
- S.Madhusudan, M.M.M.Sarcar, N.R.M.R. Bhargava, “Fabrication and Characterization of Aluminium-Copper composites”, International Journal of Alloys and Compounds, 471: 116-118 (2009).
- Wislei R. Osorio, Leandro C. Peixoto, Leonardo R. Garcia and Amauri Garcia-Corrosion behavior of hypoeutectic Al-Cu alloys in H2SO4 and NaCl solutions. Acta Metall.Sin.(Engl.Lett.) 22(4): 241-246 (2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal