The marble cutting industries are dumping the marble slurry in any nearby pit or vacant spaces, near their unit, although notified areas have been marked for dumping. This leads to serious environmental and dust pollution and occupation of vast area of land especially after the slurry dries up. This also contaminates the underground water reserves. When dumped on land, it adversely affects the productivity of land due to decreased porosity, water absorption, water percolation etc. Slurry dumped areas can not support any vegetation and remain degraded. When dried, the fine particles become air borne and cause severe air pollution. Apart from occupational health problems, it also affects machinery and instruments installed in industrial areas. During rainy season, the slurry is carried away to rivers, drains, roads, and water bodies affecting quality of water, reducing storage capacities and damaging aquatic life. Due to long term deposition on land the finer particles block flow regime of aquifers thus seriously affecting underground water availability. The heaps of slurry remain scattered all round the industrial estate are an eye sore and spoil aesthetics of entire region. Subsequently tourism and industrial potential of the state is adversely affected.
The removal of dyes in an economic fashion still remains an important problem and a number of studies have been done for the same. Panizza and Cerisola (2004) have studied the electrochemical oxidation of synthetic tannery wastewater using Ti/PbO2 and Ti/RuO2 as anodes under different experimental conditions.
Vaghela et. al (2005) have studied the reduction in COD and removal of color by the electrochemical treatment of azo dye effluent.
Asilian, H. et. al (2006) removed acrylic water base colour from synthetic wastewater using coagulation with FeSO4, alum, lime and polyelectrolyte. The obtained results showed that treatment with alum and FeSO4 alone prove to be very effective in removing colour (>99%) and part of COD (60-70%) from aqueous solution. Fongstatikul et. al (2006) explored the effectiveness of an electrochemical process to treat a sulphur dye wastewater using fine steel electrode plates. Results indicated that COD, TSS removal efficiency improved with a decrease in initial pH and increase in electrical charge.
In the present investigations, various parameters like TDS, TSS, BOD, COD, and concentration of some heavy metals have been determined in the dye effluent and then the electrochemical treatment of the dye effluent is performed using suitable electrodes and current conditions in the presence of slurry generated from stone cutting.
The present study is focused on the optimization of the electrochemical decolourisation and detoxification of the textile effluents containing reactive dyes with the aim of making this method feasible –technically and economically, at industrial scale.
Material and Methods
The dye wastewater samples were collected from various dyeing industries nearby Jaipur. The samples were analysed for various parameters like pH, TDS, BOD (270C, 3d), COD, and heavy metals. AAS technique was employed for the determination of concentration of heavy metals. The same samples were now treated electrochemically. Rectangular plates of stainless steel (SS316) were used as cathode as well as anode. Electrodes of area 4cm X 2.5 cm were immersed in the dye effluent and the electrodes were separated by a distance of 3-4 cm respectively. A Remi (2LH model) hot plate cum magnetic stirrer was used throughout the electrolysis process. A glass beaker of 1000ml capacity was used as electrolysis vessel. Electrolysis was carried out for 45 minutes. The current density was 3mA/cm2 throughout the electrolysis and 2gm NaCl was added to the one litre of the wastewater effluent. The electrolytic solution was stirred with the help of magnetic stirrer. The dye begins to precipitate and forms a loose sludge which is initially formed at the top of the mother liquor and eventually settles down at the bottom of the reaction vessel. After electrolysis the experimental electrolytic solution is filtered. The filtrate obtained was colorless and was tested for TDS, TSS, pH, BOD (270C, 3d), COD, and concentration of heavy metals.
In the second phase of the study, 250 ml slurry generated from stone cutting industry was added to one litre of dye wastewater effluent and electrochemical treatment was done using stainless steel 316 electrodes at a current density of 3mA/ cm2. Electrolysis was carried for 45 minutes. A Remi (2LH model) hot plate cum magnetic stirrer was used throughout the electrolysis process. After electrolysis the experimental electrolytic solution is filtered. The various parameters like pH, TDS, TSS, BOD, COD and heavy metal concentration were analysed again for the filtrate.
In the third phase of the study, ten Gambusia affinis were introduced in the dye wastewater effluent, electrochemically treated sample and stone slurry treated sample. These water samples were 100%, 75%, 50%, 25% concentrated samples.
Results and Discussion
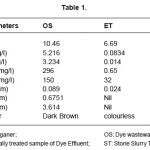
The various parameters were analysed for the dye wastewater (OS), electrochemically treated wastewater (ET) and stone slurry treated sample (ST). The results are listed in table 1.
Table 1: Parameters
The results show that the highly alkaline conditions of the OS have reduced and the pH of ET became 6.69, which is within the safe limits for ground waters. The TDS, TSS have reduced to a very large extent in ET by 98.4% and 99.56% respectively as compared to OS. BOD and COD have also reduced drastically to 0.65 mg/l and 32mg/ l respectively in ET from 296 mg/l and 150 mg/l respectively in OS.
Analysis by AAS for heavy metals shows that Cu has decreased by 26.96% after electrochemical treatment. Fe and Zn are found to be totally removed from ET sample. The dark brown colour of OS was rendered colourless in ET.
The results show that the highly alkaline conditions of the OS have reduced and the pH of ST sample became 7.89, which is within the safe limits for ground waters. The TDS, TSS have reduced to a very large extent in ST sample. BOD and COD have also reduced drastically to 20 mg/l and 10mg/l respectively from 296 mg/l and 150 mg/ l respectively in OS.
Analysis by AAS for heavy metals shows that Cu has decreased from 0.089ppm in OS to 0.015ppm in ST. Fe and Zn are found to be totally removed from ST. The dark brown colour of OS was rendered colourless in ST.
The ST sample when compared with ET sample shows that the conditions of pH are better in ST. The TDS, TSS show a decrease in ST. The BOD has shown an increase in ST but the increase is well within the safe limits. COD has shown a decrease in ST to 10mg/l from 32mg/l in ET. Cu has shown a further decrease in ST. Fe and Zn is totally absent in both the samples. Both ET and ST are colourless.
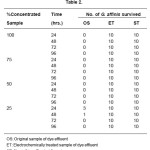
In the third phase of the study, 10 Gambusia affinis were introduced in the OS, ET and ST, which each time were 100%, 75%, 50%, 25% concentrated samples. The results are reported in Table 2.
Table 2: Concentrated Samples
It was observed that no fish could survive for more than 1.5 hours in 100%, 75%, 50% concentrated OS samples. However 30% of the fish survived for 24 hours in 25% OS concentrated sample and 10% of the fish only could survive for the next 24 hours. No fish was observed to survive after 48 hrs.
In the electrochemically treated effluent, 100% fish could survive in all concentrations of the sample. So, the effectiveness of the electrochemically treated sample can be well established.
To test the efficacy of water treated using slurry from stone industry, ten G. affinis were introduced in the ST sample.It was observed that all the animals survived for 96 hours and beyond in all concentrations of the sample ST.
Conclusion
The electrochemically treated sample showed a decrease in pH, TDS, BOD, COD, heavy metal concentration. The dark brown coloured sample was rendered colourless. Thus, the electrochemically treated water is better than the effluent sample. Further the treatment with the slurry of stone cutting industry also showed a further decrease in TDS, TSS, COD, and Cu as compared to the electrochemically treated dye wastewater sample. Fe and Zn are totally removed after the electrochemical treatment. The survival rate of Gambusia affinis was found to be 100% at all concentrations in the stone slurry treated dye wastewater sample. This clearly establishes the role of stone slurry in the treatment of dye wastewater.
References
- Khan T.I., Kaur N., Vyas P., Effect of industrial effluents on physico-chemical characteristics of the Amanishah Nallah, Journal of Env. And Poll., 2(3): 147-150 (1995).
- Nanda, P., Panigrhi, S., Nanda, B. and Behera, M.K., Toxicity of paper mill effluent to fishes. Env. Eco., 18(1): 220-222 (2000)
- Singh, R.B., Cytotoxic effect of synthetic blue dye used by green grocer, J. Env. Polln. 7(2): 77-81 (2000)
- Sridevi, B. and Reddy, S.L.N., Effect of trivalent and hexavalent chromium on carbohydrate metabolism of a fresh water field crab Barytelphusa guerini, Environ. Monit. Assess, 61(2): 291-300 (2000)
CrossRef
- Panizza, M. and Cerisola, G., Electrochemical oxidation as a final treatment of synthetic tannery wastewater. Environ. Sci. Technol. 38(20): 5470-5 (2004)
CrossRef
- Chen X., Shen Z., Zhu X., Fan Y., Wang W.,Advanced treatment of textile wastewater for reuse using electrochemical oxidation and membrane filtration, Water SA, 31(1): 127-131, (2005)
CrossRef
- Ghalwa, N.M.A. and Latif, M.S.A, Electrochemical degradation of acid green dye in aqueous waste water dyestuff solutions using a lead oxide coated Ti electrode, Journal of the Iranian Chemical Society, 2(3): 238-243 (2005)
- Panizza M., Bocca C. and Cerisola G., Electrochemical treatment of wastewater containing polyaromatic organic pollutants, Water research, 34(9): 2601-2605 (2005).
CrossRef
- Vaghela S.S., Jethva A.D., Mehta B.B., Dave S.P., Adimurthy S., Ramachandraiah G., Laboratory studies of electrochemical treatment of industrial azodye effluent, Environ. Sci. Technol., 39(8): 2488-55 (2005).
CrossRef
- Asilian H., Moradian F., Rezaei A., Mortazani S.B. and Khavanin, A., The removal of colour and COD from wastewater containing water base colour by coagulation process. Int. J. of Env. Sc. and Tech. (CEERS), 3(2): Spring. 153-157 (2006).
CrossRef
- Fongsatitkul, P., Elefsiniotis, P. and Boonyanitchakul, B., Treatment of a textile dye wastewater by an electrochemical process, J. Env. Sc. and Health. 41(7): 1183-1195 (2006).
CrossRef
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal