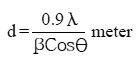Effects of Organic Additive Saccharin on the Magnetic Properties of Comnp Thin Films
M.RM. Krishnappa1*, N. Rajasekaran2, S. Ganesan3 and R.N. Emerson4
1Depatment of Physics, Sri Ramakrishna Engineering College, Coimbatore - 641 022, India.
2Depatment of Chemistry, Sri Ramakrishna Engineering College, Coimbatore - 641 022, India.
3Depatment of Physics, Government College of Technology, Coimbatore - 641 013, India.
4Depatment of Physics, Government Arts College, Udhagamandalam - 643 002, India.
DOI : http://dx.doi.org/10.13005/msri/070119
Article Publishing History
Article Received on : 20 Apr 2010
Article Accepted on : 24 May 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Electrodeposition technique provides an easy way to produce magnetic thin films. Magnetic thin films are extensively used in various magnetic induction writing heads, magnetic MEMS devices and other magnetic sensing devices. Thus we have synthesized CoMnP thin films from aqueous bath with organic additive saccharin using electrochemical deposition technique and examined its magnetic properties. The electrochemical deposition method is especially interesting due to its low cost, high throughput and high quality of deposit. The CoMnP alloys were electrodeposited galvanostatically for various composition of the bath solution and for various concentration of the additive saccharin. The effects of electrodeposition condition and organic additive saccharin on the magnetic properties of CoMnP thin films were investigated. Structure and the Morphology of the film were studied using X-Ray diffractometer (XRD) and scanning electron microscopy (SEM). Elemental compositions of the film were studied using energy dispersive X-Ray Spectroscopy (EDS). Magnetic properties of the deposited films were studied using Vibrating sample Magnetometer (VSM). SEM measurement indicated that the surface morphology was affected by the nature of the organic additives to a large extent. CoMnP films formed under optimized conditions are found to be polycrystalline in nature with hcp structure. Moreover, it was obvious that the presence of organic additive saccharin, in the electroplating bath, modified the magnetic properties of the CoMnP thin films according to the VSM measurements.
KEYWORDS:
CoMnP thin films; Organic additive saccharin; Current density; Magnetic saturation; Coercivity.
Copy the following to cite this article:
Krishnappa M. MR, Rajasekaran N, Ganesan S, Emerson R. N. Effects of Organic Additive Saccharin on the Magnetic Properties of Comnp Thin Films. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Krishnappa M. MR, Rajasekaran N, Ganesan S, Emerson R. N. Effects of Organic Additive Saccharin on the Magnetic Properties of Comnp Thin Films. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2261
|
Introduction
Magnetic thin films have received increasing attention over last decade due to their potential application in magnetic induction writing heads,1-5 magnetic MEMS devices6,7 and other magnetic sensing devices. Co base alloy thin films are promising candidates for high density magnetic recording media. Co based thin films can be prepared by the chemical deposition technique which make them particularly attractive for commercial application since the deposition rate is much faster than the other methods. It is well known that the magnetic properties of these film strongly depend on the micro structure, features such as grain size, film thickness and crystallographic texture.8-12 The micro structure is controlled by the deposition conditions such as the pH, current density and temperature of the plating bath. Hence there are several detailed studies which correlate the magnetic properties and the deposition conditions.13,14 Various studies have been carried out to develop magnetic thin films, which have hard magnetic properties.15 Numerous studies have been carried out to investigate binary and ternary Co based iron group magnetic thin films, they mostly focused on the mechanism of anomalous codeposition, the effect of various additive, effect of plating and the corrosion properties. To our knowledge, there have been few detailed studies on Co based films prepared using electrodeposition.16-19 The present work was to study the effect of electrodeposition conditions and additives on the magnetic properties of CoMnP thin films.
Material and Methods
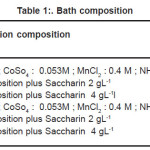
CoMnP thin films were electrodeposited from chloride baths. Table I lists the plating solution composition investigated. Analytical reagent grade chemicals were used to prepare baths. Solution pH was adjusted to 3 with either HCl, H2So4 or NaOH. CoMnP thin films were electrodeposited at room temperature without stirring. The effect of NaH2Po2 on the deposited composition was investigated for two different concentrations at 0. 2M and 0.4 M. Thin copper substrate of 20mm(width ) X 130 mm ( length ) X 0.1 mm (thickness) were used as anode for electrodeposition experiments. Current for electrodeposition was passed from a regulated direct current unit. Before electrodeposition these substrates were electrocleaned in a alkaline electrocleaning bath. The bath contained Sodium hydroxide: 7.0 gL-1; Sodium carbonite 20.0 gL-1; trisodium phosphate: 9.0 gL-1; and Sodium metasillicate: 24.0 gL-1. The bath was operated at 3. 0 A dm -2 After electrocleaning, the substrates were rinsed in distilled water. The CoMnP alloys were electrodeposited for various composition of the bath solution A1, A2, B, B1, B2 and for various concentration of the additive saccharin. CoMnP films were electrodeposited at constant pH value 3 by varying the current densities ( 3,5 and 7 mA-cm -2 ) for various time durations ( 15,30 and 60 Minutes ). The thickness of the deposition was tested using digital micrometer. Magnetic properties of the depositied films were studied using Vibrating sample Magnetometer ( VSM ).X-Ray diffractometer ( XRD ) was used to study the crystal structure and grain size. Scanning electron Microscope ( SEM ) was used to study the morphology of these magnetic films.
Results and Discussion
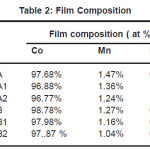
Elements present in the film were analyzed by energy dispersive X-ray spectroscopy (EDS) and the results are shown in Table 1. It was observed that all the films obtained from various baths had less than 2.0% phosphorous. Even with low phosphorous content the films showed high magnetic properties. The improved crystalline structure of CoMnP films was due to the addition of saccharin in the various baths. Elements present in the film were analyzed by energy dispersive X-ray spectroscopy and the results are shown in table 2.
Table 1: Bath composition
Table 2: Film Composition
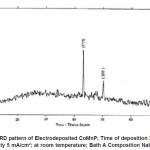
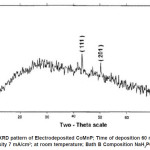
Electro deposited CoMnP films were subjected to XRD studies. X-ray Diffraction patterns of various CoMnP electrodeposits produced from various current densities 3, 5 and 7 mA cm-1 and various time of depositions 15, 30 and 60 minutes were obtained. The XRD pattern is presented in the Fig. 1 and Fig. 2. The intensity data obtained was from 20 °C to 80 °C. The XRD datas obtained are compared with Joint Committee for Powder Diffraction Data (File No. 71 / 2336). CoMnP films had Hexagonal Close Packed (hcp) structure and exhibited (201) plane primarily. (201) plane peaks in the data for films obtained for bath A and B has shifted because of the film stress. From the previous studies12, it is learnt that the film stress will shift XRD peaks. The crystalline sizes of the deposits were calculated from Debye – Scherrer formula.
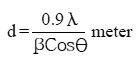
Where, λ is the wavelength of radiation. λCu = 1.54056 Å. β is full width at half maximum (FWHM in radians). è is the position of the maximum diffraction. The values obtained clearly shows that the crystalline sizes are in nanoscale. (111) and (201) Plane peak in the data for films obtained from bath A and bath B were shifted because of film stress. From literatures, It is known that film stress will shift XRD peaks.12 Stress was low for bath A1 and B1 which contains 2 gL-1 of saccharin. It increased on increasing the concentration of saccharin to 4 gL-1 in the baths A2 and B2. It is because of incorporation of decomposed products of additive in to the film.
Figure 1: XRD pattern of Electrodeposited CoMnP; Time of deposition 30 min; Current Density 5 mA/cm2; at room temperature; Bath A Composition NaH2PO2 : 0.2 M
Figure 2: XRD pattern of Electrodeposited CoMnP; Time of deposition 60 min; Current Density 7 mA/cm2; at room temperature; Bath B Composition NaH2PO2 : 0.4 M
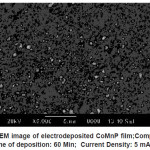
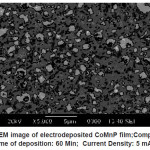
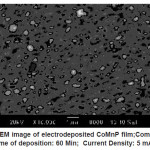
Electrodeposited CoMnP films for all the bath compositions were subjected to SEM studies. The monographs are shown in Fig. 3 – 5. Fig. 3 shows the SEM image of electrodeposited CoMnP film; Composition : Bath A; Time of deposition: 60 Min; Current Density: 5 mAcm-2; pH: 3. Figure IV shows the SEM image of electrodeposited CoMnP film; Composition : Bath A1;Time of deposition: 60 Min; Current Density: 5 mAcm-2; pH: 3. Figure V shows the SEM image of electrodeposited CoMnP film; Composition : Bath A2; Time of deposition: 60 Min; Current Density: 5 mAcm-2; pH: 3. The film with very low concentration of saccharin appeared to have a crevice pattern. The film obtained from a bath contained 4 gL-1 of saccharin was cracked through substrate due to the stress of the film as shown in the figure V. In general, micrograph of the CoMnP film is greatly influenced by the additive saccharin and current density. Adhesion of the film with the substrate is tested by bend test and scratch test. It is found that film is having good adhesion with the substrate.
Figure 3: SEM image of electrodeposited CoMnP film;Composition: Bath A; Time of deposition: 60 Min; Current Density: 5 mAcm-2; pH: 3
Figure 4: SEM image of electrodeposited CoMnP film;Composition : Bath A1; Time of deposition: 60 Min; Current Density: 5 mAcm-2; pH: 3
Figure 5: SEM image of electrodeposited CoMnP film;Composition: Bath A2; Time of deposition: 60 Min; Current Density: 5 mAcm-2; pH: 3
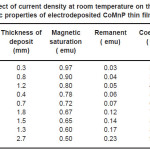
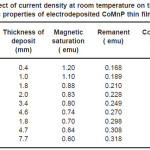
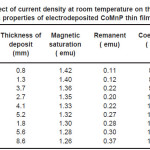
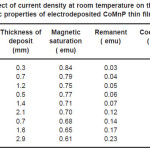
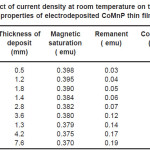
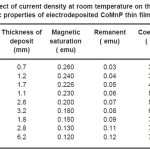
Electrodeposition studies are carried out for different bath compositions and for different bath conditions. Table III to VIII shows the results of electrodeposition of CoMnP and their magnetic properties. The thickness of the film for various current densities and time of deposition are also shown in table III to VIII. The magnetic properties of the film were found to increase with current density and duration of deposition. The effect of addition of saccharin in to all the baths along with NaH2PO2 was investigated. The magnetic properties of the thin film improve significantly upon adding low concentration the additive saccharin. Under the best condition involving addition of 0.2M of NaH2PO2 and 2 g-l-1 of saccharin at a current density of 7 mA-cm-2 and time of deposition 60 minutes, the thickness of the film was found to be 7. 7 micrometer with coercivity 1700 Oe and remanent 0.318 emu. With further increase in NaH2PO2 concentration, the thickness of the film found to be 7.6 micrometer with coercivity value of 950 Oe and remanent value of 0.19 emu. Increase in magnetic properties of the films is mainly due to saccharin. The electrodeposited films were uniform and bright. The saccharin molecules are found to have leveling effect, which ensures uniform orientation of crystals during electrodeposition. On increasing the concentrartion of NaH2PO2 and saccharin, the magnetic properties of the film decreased because of the stress present in the films. Stress is caused by the inclusion of decomposed products of additives.
Table 3: Effect of current density at room temperature on the thickness and magnetic properties of electrodeposited CoMnP thin films for bath A
Table 4: Effect of current density at room temperature on the thickness and magnetic properties of electrodeposited CoMnP thin films for bath A1
Table 5: Effect of current density at room temperature on the thickness and magnetic properties of electrodeposited CoMnP thin films for bath A2
Table 6: Effect of current density at room temperature on the thickness and magnetic properties of electrodeposited CoMnP thin films for bath B
Table 7: Effect of current density at room temperature on the thickness and magnetic properties of electrodeposited CoMnP thin films for bath B1
Table 8: Effect of current density at room temperature on the thickness and magnetic properties of electrodeposited CoMnP thin films for bath B2
Conclusions
CoMnP thin films could be successfully deposited by electrochemical deposition technique. Effect of deposition condition and saccharin concentrations in the starting solution ( Bath A and B )on the formation of CoMnP thin films was investigated. The concentrations of the constituents in the starting solution were optimized using a Hull cell. CoMnP films had Hexagonal Close Packed (hcp) structure and exhibited (201) plane primarily. Under the best condition involving addition of 0.2M of NaH2PO2 and 2 g-l-1 of saccharin at a current density of 7 mA-cm-2 and time of deposition 60 minutes, the thickness of the film was found to be 7. 7 micrometer with coercivity 1700 Oe and remanent 0.318 emu.. (201) Plane peak in the data for films obtained from bath A and bath B were shifted because of film stress. From literatures, It is known that film stress will shift XRD peaks12. Stress was low for bath A1 and B1 which contains 2 gL-1 of saccharin. It increased on increasing the concentration of saccharin to 4 gL-1 in the baths A2 and B2. It is because of incorporation of decomposed products of additive in to the film.. The CoMnP alloy deposited from the chloride bath can further be studied for its corrosion properties. CoMnP thin films can be synthesized from various additives such as urea and thiourea using electrochemical deposition technique and its magnetic properties can be examined for better magnetic properties.
Acknowledgements
The authors thank the management of Sri Ramakrishna Engineering College for their support and kind permission to publish this paper.
References
- P.C. Andricacos, N. Robertson, IBM J. Res.Develop, 42: 671 (1998)
CrossRef
- E.I. Cooper, C. Bonhote, J. Heidmann, Y.Hsu,P.Kern,J.W.Lam,M.RamaSubramanian, N. Robertson, L. T.
Romankiw, H. Xu, IBM J. Res. Develop. 49: 103 (2005)
CrossRef
- E.Gomez, E.Pellicer, E. Valles, Electrochem Commun 7: 275 (2005)
CrossRef
- K.Ohashi,N.Morita,T.TsudaY. Nonaka IEEE Trans.Magnn 35: 2538 (1999)
CrossRef
- T.Osaka, Electro Chem. Acta 44: 3885 (1999)
CrossRef
- J.Y.Park,M.Allen, J. Micromech. microeng 8:307(1998)
- S.Guan, B.J.Nelson, J. Electrochem. Soc152: 190 (2005)
CrossRef
- J.S.Judge,J.R.Morrison and D.E Speliotis J.Electro Chem. soc 113: 547 (1966)\
CrossRef
- M. J. Miksic, R. Travieso, A. Arcus and R. H.wright, J. Electro Chem. Soc 113: 360 (1996).
CrossRef
- J. S. Judge, J.R. Morrison and D.E Speliotis J. Appl. Phys 36: 948 (1965).
CrossRef
- M. Aspland, G.A. Jones and B.K. Middleton, IEEE Trans. Magn. MAG , 5: 314 (1969).
CrossRef
- T. Chen, D.A. Rogowski and R.M. White J. Appl. Phys 49: 1816 (1977).
CrossRef
- F. E. Luborsky, J.Appl. Phys 32: 171 (1961).
CrossRef
- M. Hansen, Constitution of Binary Alloys, Mc Graw – Hill, NewYorK, p. 489 (1958).
- C. Byun, G. C. Rauch, D. J. Young C.A. Klepper Greggi Jr. J. Appl. Phys 73(10): 5575 (1993).
CrossRef
- T. M. Liakopouios, W. Zhang and CH. AGN, IEEE Trans, Magn 32(5): 5146 (1996)
- V.V. Bonder, M.M. Mel’nikova and Y.V. M. Polukarov “ Electrodeposition of metals and alloys” N. E. KHOMUTOV Eds. IPST Press,71- 109 (1969).
- J. Horkans, D. J. Seagle and I. Chia Hsu Chang, J. Electro Chem. soc 109: 485(1962).
- T.M. Liakopoulos, W. Zhang and C. H. AGN IEEE Trans. Magn. 32(5): 5146 (1996).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal