Electrochemical Preparation and Characterization of Ni Deposition on Aluminum Surface
R. K. Pathak2, Priyanka Wagela2, S. Gohar2 and P. Ramshankar1*
1RRCAT, Indore, India.
2Department of Chemistry, Govt.Holkar Science College, Indore, India.
DOI : http://dx.doi.org/10.13005/msri/070137
Article Publishing History
Article Received on : 29 Jan 2010
Article Accepted on : 09 Mar 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The electrochemical preparation and characterization of Ni deposition was performed on aluminum surface. The Aluminium electrode was anodically oxidized to obtain a porous alumina template. The properties of various porous Al oxide films were compared in terms of their electrochemical responses during Ni eletrodeposition. The corrosion behaviour of deposits was evaluated by polarization curves in 0.1 M NaCl solution at room temperature. The surface morphology of deposited films were investigated by scanning electron microscopy (SEM). It has been found that the deposition of Ni on Al surface disturbs the Aluminum coating's regular surface structure. The compositional analysis of electrodeposited films was investigated by Energy dispersive X-ray spectroscopy (EDAX).
KEYWORDS:
Electro deposition; Polarisation
Copy the following to cite this article:
Pathak R. K, Wagela P, Gohar S, Ramshankar P. Electrochemical Preparation and Characterization of Ni Deposition on Aluminum Surface. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Pathak R. K, Wagela P, Gohar S, Ramshankar P. Electrochemical Preparation and Characterization of Ni Deposition on Aluminum Surface. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2323
|
Introduction
Thin film surface coating are widely used for efficient conversion of solar radiation into useful forms. One of the most frequently and commercial material in solar absorbance is nickel pigmented aluminium oxide on aluminium.A simple and inexpensive technique for the preparation of thin film is electrochemical deposition. It has been used successfully in the case of Ni for a wide range of concentration of metal. Theproperties, the composition and the grain size of electrodeposited Ni are strongly dependent on the electrolyte composition and the deposition parameters. The objective of the present work has been focused on the development of Ni thin film, electrodeposited on anodized alumina surface and their characterization. The structural morphology and composition analysis of the electrodeposited thin film were performed by SEM and EDAX. The corrosion behavior of electrodeposited Ni in NaCl solution have also been studied.
Experimental
The anode of the electrochemical cell was an aluminum plate while the cathode was lead plate. Firstly the aluminum plate is converted into anodic oxide by anodization. For this process we use 10% oxalic acid as anodizing solution. Temprature is maintained between 18-200C. The lead plate is immersed in anodizing solution for ½ hr, then Al plate is immersed in this solution. (Voltage-30V, Time-1 hr). In this process H2 gas is produced which goes towards anode and form bubble at lead plate. Oxygen goes towards Al plate and makes it anodic oxide. All electrochemical experiments and the deposition processes were carried out in a standard three electrode electrochemical cell equipped with platinum net as a counter electrode and saturated calomel electrode (SCE) as a reference electrode.
The plating time was 30 sec after which the anode was withdrawn, washed with distilled water and dried. All solutions were prepared with analytical grade chemical reagents and deionised water.Ni/Al film was obtained by the electrodeposition from the solution shown in table 1
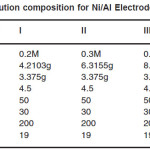
Table 1: Solution composition for Ni/Al Electrodeposition
The corrosion behaviour of deposited films were evaluated by polarization curves. With the help of tafel plot we have determined Corrosion current, corrosion potential and corrosion rate. The electrochemical behavior of the electrodeposited specimens was analyzed in a 0.1 M NaCl aqueous solution at room temperature. The surface morphology of deposited films was studied by SEM.The compositional analysis of electrodeposited films was investigated by EDAX.
Results and Discussion
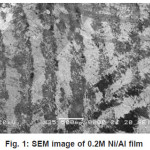
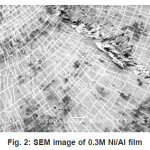
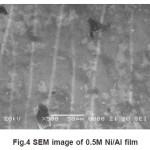
The morphological characterisation were studied by SEM.These investigation are limited by resolution of the SEM, satisfactory quality of SEM image was possible when the thickness of single Ni layer exceeds-300 µ. Based on SEM investigation high efficiency of the deposited thickness measured by SEM was similar to that predicted from deposition charge. Some of the SEM images of deposited films are shown in figure 1 to 4. The visible areas where single Ni layers seem to be not continuous are caused by some difficulties in etching during sample preparation.
Figure 1: SEM image of 0.2M Ni/Al film
Figure 2: SEM image of 0.3M Ni/Al film
Figure 3: SEM image of 0.4M Ni/Al film
Figure 4: SEM image of 0.5M Ni/Al film
All the deposits obtained from the investigated solution adhere well to Aluminum oxide substrate. The metallic luster and brightness of the deposit decreased with presence of Ni. It was observed that the deposits are generally composed of fine grains.The presence of microcracks is probably due to the growth of internal stresses during the electrodeposition..
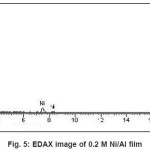
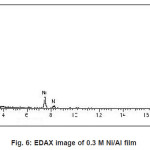
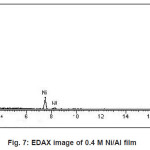
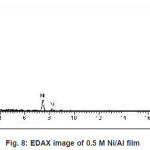
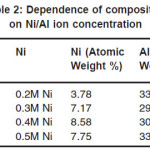
Figure 5 to 8 shows the EDAX analysis of the electrodeposits of different concentration of Ni. The EDAX analysis confirms the inclusion of Ni in the deposits. Table 2. Shows the atomic and weight percent of Ni in electrodeposits.
Figure 5: EDAX image of 0.2 M Ni/Al film
Figure 6: EDAX image of 0.3 M Ni/Al film
Figure 7: EDAX image of 0.4 M Ni/Al film
Figure 8: EDAX image of 0.5 M Ni/Al film
Table 2: Dependence of composition on Ni/Al ion concentration
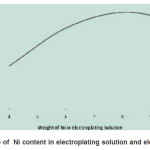
Figure 9 shows the relation between the weight of Ni in electroplating solution and in electrodeposited films.This confirms that when the concentration of Ni increases in solution, the Ni deposits also increases in the films.
Figure 9: Dependance of Ni content in electroplating solution and electrodeposited film
Tafel polarization was performed to evaluate the corrosion parameter of the deposits with different Ni contents. A small current plateau is observed in the tafel curve of the Ni/Al coating , which could be associated with accelerated passivation process. The corrosion current density values were estimated making use of tafel slope method.The corrosion potential and corrosion current for the Ni/Al coating,obtained from polarization curves. A representative tafel plot is shown in figure 10. As shown in the plot, the corrosion current of the deposits decreases with increase in the amount of Ni. Hence corrosion resistance increases at the higher concentration of Ni in the deposited films.
Figure 10: Tafel plot of 0.2M Ni/Al film
Acknowledgements
The authors are grateful to RRCAT Indore and Dr.D.M.Phasey IUC DAVV, Indore for instrumental analysis. The Authors would also like to thanks Prof. A.K.Silawat,HOD, chemistry of Gov.Holkar Science College, Indore for their support.
References
- Giouroudi, Ktena A., E.Hristoforou 2004 J.Opto. and Adv.Materials 6 :661-666( 2004).
- Costa Kieling V., Surface Coating Technology 96: 135 (1997).
- H.Li, Ebrahimi H., Mater. Sci. Eng. A 347:93 (2003).
- Ovari T.A., Tibu M. and Chiriac H., Sensors Actuat. A106: 267 (2003).
- Georgescu V., Georgescu M., Surface Science 507 (2002).
- Georgescu V., Georgescu M., J. Magn.Magn.Mater. 242-245, 416 (2002).
- Pedro De Lima-Neto, Correia A.N.,Colares R.P., Araujo W.S. J.Braz. Chem. Soc. 18: (2007).
- Da Silva, P.S.G, Costa, A.N.C, Mattos, O.R.,Correia, A.N.,De Lima-Neto, P., J. Appl. Electrochem. (2006).
- Jayalakshmi M., Woo-Young Kim, Kwang-Deog Jung, Oh-Shim Joo, J. Electrochem. Sci, 3: 908-917 (2008).
- Bicelli L.P., Bozzini B., Mele C., Urzo L.D., Int. J. Electrochem. Sci. 3: 356 (2008).
- Jayalakshmi M., Muralidharan V.S., Br. Corrosion J. 26: 123 (1991).
- Jayalakshmi M., Indra Puspitasari, Kwang-Deog Jung, Oh-Shim Joo, Int. J.Electrochemical. Sci. 3:787 (2008).
- Kadirgan F., Wackelgard E., Sohmen M., Turk J Chem., 23:381-391 (1999).
- Tokarz A., Fraczek T.,Balaga Z.,Nitkiewicz Z.,Rev. Adv. Mater. Sci. 15: 247-252 (2007) .
- Gomez E.,Pane S., and E.Vallas, J. Electrochem. Sci. Acta 51: 146 (2005).
- Chassaing E., J. Electrochem. Soc. 144: L328 (1997).
- Landolt D. and Marlot A., Surface and Coating Technology., 169 (2003) .
- Karahan I. H., Chinese J. Physics, 46:1 (2007).
- Afshar A., Dolati A.G. and M. Ghorbain, Mater.Chem.Phys. 77: 352 (2002).
- Leith S.D., Ramli S. and Schwartz D.T., J. Electrochem. Soc. 146: 1431 (1999).
- Leith S.D., Wang W. and Schwartz D.T., J. Electrochem. Soc. 146: 2827 (1999).
- Short N.R., Zhou S. and J.K.Dennis, Surf.Coat.Tech. 79, 218 (1996).
- Ramanauskas R., Appl. Surf. Sci.153: 53 (1999).
- Karahan I.H., J. Mater. Sci. 24: 10160 (2007).
- Gurrappa I. and Binder L., Sci. Tech. Adv. Mater. 9: 043001 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal