B. K. Pandey1, S. P. Dubey2* and G. K. Gupta1
1Department of Applied Sciences, M.M.M.Engineering College, Gorakhpur, India.
2Department of Physics, M.G.P.G College, Gorakhpur, India.
DOI : http://dx.doi.org/10.13005/msri/070143
Article Publishing History
Article Received on : 01 Jan 2010
Article Accepted on : 01 Feb 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The behaviour of dielectric constant with the variation of temperature of barium nitrate has been studied between the temperature ranges 35 to 110ºC. In the present measurement it have been observed that the compound has lower value of dielectric constant below 36ºC, which rises upto the maximum value of 14,580 at the moderate temperature of 51ºC. After this temperature the dielectric constant of compound decreases upto the very low value of nearly 8, at the temperature of 108ºC. When the variation of dielectric constant was studied in cooling cycle the peak was observed at 71ºC, above and below this temperature dielectric constant decreases with some intermediate fluctuations. The results have been explained on the basis the crystal structure changes and the possibility of free internal rotation of nitrate group within the crystal lattice at the elevated temperature. The presence of broad peak is explained on he basis of relaxor ferroelectrics, which occurs generally due to heterovalent disorder.
KEYWORDS:
Barium nitrate; Feroelectricity; Phase transition
Copy the following to cite this article:
Pandey B. K, Dubey S. P, Gupta G. K. Ferroelectric Behaviour and Phase Transition of Barium Nitrate. Mat.Sci.Res.India;7(1)
|
Introduction
Owing to the extraordinarily structure sensitive character of the phenomenon of feroelectricity, it was only logical that, after the discovery of ferroelectricity in BaTiO3, considerable effort would be expanded on a search for other ferroelectric materials with in the same family. This search was definitely fruitful, as it led to the discovery of ferroelectric activity in PbTiO3, KNbO3 and KTaO3.These compounds have curie points scattered over a wide range of temperatures, while the room-temperature symmetry is characterized by very different distortions from the original perovskite cubic lattice.
The general formula of the compounds belonging to this family is ABO3 where A is monovalent, divalent or trivalent metal and B a pentavalent, tetravalent or trivalent element respectively. We include in the perovskite family not only the compounds having the ideal cubic perovskite structure, but also all the compounds with structures, which can be derived from the ideal one by way of small lattice distortions or omission of some atoms. The crystal-chemical characteristics of perovskite-type compounds in general have been studied.7, 5, 9
Our sample Ba(N03)2 lie in the range of perovskite-type oxides family. The variation of dielectric constant can be explained on the basis of the arrangement of ions in the compound and its rotation inside the crystal lattice under influence of thermal energy. It is observed that the high value of dielectric constant in ferroelectric phase is due to high polarization. The temperature dependence of dielectric constant is explained on the basis of activated polarization process due to the phase transition in the compound and the occurrence of broad peak has been explained on the basis of relaxor system, as it is repoted,12, 13 in the case of disodium hydrogen ortho-phsphate and for tungsten- bronze type compound.2,10, 14, 1
Experimental
The compound has been procured from E. Merk (India), Mumbai. The chemical was grinded into the fine powder in a agate mortar, avoiding direct sunlight and preferably the most of the sample preparation was done at night. The pellets were prepared with compression machine (Flextural Testing Machine CAT No.AIM-313, S.No.91070 AIMIL Associated, India), having pressure range 0-10 tonne wt/cm2. A suitable die was used having rectangular cross-sectional area of the piston = 2.33cm2.
The polishing of the pellets has been done to obtain smooth parallel surface to be used for electrode formation. Polishing of the crystal introduces electrical charges inside the material. These charges and strains are to be removed, which we did by the process of annealing of the sample. In this process the pellets were kept in a suitable furnace at nearly 2/3 of their melting points for sufficient times (generally 8-10 hours). The most of the irreproducibility was removed by annealing and therefore this process was necessarily done. The electrodes were formed using colloidal silver paints.
The sample holder loaded with pellet is kept into the furnace such that it lies very near to the middle part of the furnace. A good quality thermometer, precisely calibrated is used to record the temperature. This thermometer is adjusted with the help of stand in such a way that it touches the metallic part of sample holder to record the exact temperature of sample.
The usual substitution method i.e. with and without the specimen in suitable sample holder is used.4 The sample holder was directly fastened to the capacity measuring unit (Zenith M 92A).
Results and Discussion
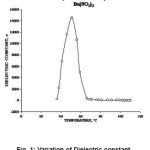
The compound has chemical formula Ba(N03)2 with molecular weight 261.35. The solid is colourless cubic crystal having refractive index 1.572 with density 3.24 gm/cm3. Due to high M.P. (590°C) the measurement may be taken upto higher temperatures safely.8 However, during heating cycle of the study, the dielectric constant was 278 at the room temperature of 36°C due to the phase transition. The dielectric constant of this compound increased to very high value rapidly and attained the value of 14580 at the moderate temperature of 51ºC and then starts to fall slowly and finally approached to a very low value of nearly 8 at the temperature of 108ºC.3 The data has been shown in the Fig.1.
Figure 1: Variation of Dielectric constantwith Temperature of Ba (NO3)2
The behaviour of dielectric constant of Ba(NO3)2 with the variation of temperature can be explained in context to its structural phase change and rotation of nitrate groups within the crystal lattice at elevated temperatures, similarly as it is in case of Lead Nitrate.12, 13 The crystal structure of solid barium nitrate is in such a way that the compound crystallizes in the cubic system with the lead atoms in a face centered cubic system. The two nitrate groups lie above and below barium atoms at the same distance to the plane containing barium atoms. From the Fig.1, it is clear that there is no sharp change in the dielectric constant at transition temperature but it have broad peak, which appears in the temperature range 45ºC to 55ºC in heating cycle. The appearance of broad peak can be explained on the basis of relaxor ferroelectrics.11 The temperature dependence of dielectric constant of Ba(NO3)2 shows a broadened peak, unlike that of normal ferroelectrics at the curie point. When the temperature is higher than Tm (the temperature at which the dielectric constant is maximum i.e. ε m) ε (T) does not obey the Curie-Weiss law exactly. In fact ε (T) changes with in the following fashion.
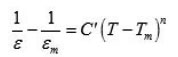
Where 1<n<2 and C’ is a constant
Before the Tm, as temperature is increased from room temperature the dipoles got sufficient kinetic energy to face hindrance, in rotation and alignment. The dielectric constant and the alignment of dipoles could not achieve the previous high value of å at the moderate temperature of 54ºC in cooling cycle. In the same cycle, the dielectric constant has its highest value again, in the same range of temperature at 71ºC, which is moderately different than that of heating cycle. The peak value of the dielectric constant at the Curie temperature (71ºC) as observed in our sample (Fig.2) and the shape of the anomaly are strongly dependent on the rate of temperature change during the measurement. The shape of the anomaly depicted in cooling cycle is obtained with very slow cooling rates and due to the structural phase change in the crystal. The fluctuation in the variation of dielectric constant with temperature is due to the rotation of ions in the crystal lattice of the compound. The presence of broad peak can be explained in the similar way as in the relaxor ferroelectrics. The variation of å with temperature in our sample is similar to those obtained for single crystal of LiNH4C4H4O6.H2O.6
Figure 2: Variation of Dielectric constant with Temperature of Ba (NO3)2
Conclusion
In this work we have concluded that the change in temperature changes the structure causing the phase change in the compound and dipoles are created which get sufficient kinetic energy to face hindrance. The rotation and alignment of the dipoles shows the ferroelectricity in the compound. The presence of broad peak is explained similar to the relaxor systems, which are generally due to heterovalent disorder.
Acknowledgements
The authors are thankful to Head Department of Physics D.D.U.Gorakhpur University, Gorakhpur, the Principal, M.M.M. Engineering College, Gorakhpur for providing experimental facilities and their encouragements.
References
- Chen, Z. L., Yao, X. and Cross, L.E., Ferroelectrics, 49: 213 (1983)
- Cross, L.E., Ferroelectrics, 76: 241 (1987)
- Dubey, S. P., D. Phil Thesis, “The Study of Crystal Structure and Ferroelectricity” Submitted H. N. B. Garhwal University, Srinagar (2009)
- Gupta, R. N. and Mishra, M., India J. Pure Appl. Phys., 19: 1151 (1981)
- Megaw, H.D., Proc. Phys. Soc. (London), 58: 10 (1946)
- Merz, W.J., Phys. Rev., 82: 562 (1951)
- Naray-Szabo, S., Naturwiss, 31: 202, 466 (1943)
- Patnaik, Pradyot., Handbook of Inorganic Chemicals, ISBN 0070494398 ‘McGrawHill (2002)
- Roth, R.S., J. Research Natl. Bur. Standards., 58: 75 (1957)
- Setter, N and Cross, L. E., J. Appl. Phys., 51: 4356 (1980)
- Smolenskii, G. A., Isupov, V.A., Agranovskaya, V. A. and Krainik, N. N., Sov. Phys. solid state, 2: 2584 (1961)
- Yadava, S., Pandey, B. K., Dubey, S. P. and Gupta, R. N., Asian J. Chem., 20(1): 731-735 (2008)
- Yadava, S., Pandey, B.K., Dubey, S. P. and Seth, J. P., Asian, J. Chem., 20(3): 2051-2056 (2008)
- Yao-X, Chen, Z. L. and Cross, L.E., J. Appl. Phys., 54: 3395 (1984).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal