Immobilization of a - Amylase from Locale Bacteria Isolate Bacillus Subtilis ITBCCB148 with Diethylaminoethyl Cellulose (DEAE-Cellulose)
Yandri, Tati Suhartati and Sutopo Hadi
Department of Chemistry, Faculty of Mathematics and Natural Sciences University of Lampung, Bandar Lampung-35145, Indonesia.
DOI : http://dx.doi.org/10.13005/msri/070114
Article Publishing History
Article Received on : 15 Apr 2010
Article Accepted on : 26 May 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The thermal stability increase of a-amylase obtained from locale bacteria isolate Bacillus subtilis ITBCCB148 was achieved by immobilization process using an ionic exchange matrix of DEAE-Cellulose. The result showed that the immobilized enzyme has an optimum temperature of 60°C; KM 14.8 mL substrate and Vmax 42.4 U/mL. The thermal stability storage temperature of 60°C, pH 9.0 and 60 minutes demonstrated the immobilized enzyme has residual activity of 28.1%; ki = 0.0224 min.-1; and ΔGi = 103.7 kJ mol-1. Although the immobilized enzyme’s thermal stability was only increased 1.5 times, at higher temperatures, it was much more stable than the native enzyme.
KEYWORDS:
α-amylase; Immobilization; DEAE-Cellulose; B. subtilis ITBCCB148
Copy the following to cite this article:
Yandri, Suhartati T, Hadi S. Immobilization of a - Amylase from Locale Bacteria Isolate Bacillus Subtilis ITBCCB148 with Diethylaminoethyl Cellulose (DEAE-Cellulose). Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Yandri, Suhartati T, Hadi S. Immobilization of a - Amylase from Locale Bacteria Isolate Bacillus Subtilis ITBCCB148 with Diethylaminoethyl Cellulose (DEAE-Cellulose). Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2245
|
Introduction
α -Amylase (α-1,4-glucan-4-glucanohidrolase, EC 3.2.1.1) is an enzyme that catalyzes the bond breaking of α-D-(1,4)-glucosidic bonds in amylum, glycogen and oligosaccharides. This enzyme is widely applied in many industrial processes, such as the production of sugar syrup. In order to be used in industrial sectors, α-amylase must accomplish some requirements such as having high thermal stability and wider working pH.1
Few methods are usually applied in attempts to increase the stability of native enzymes, these include the immobilization process, chemical modification and directed mutagenesis.2 The use of enzyme immobilization in industrial processes are still widely applied since there are some benefits of using it, other than that it can be used frequently and continuously, the immobilization process also increases the enzyme stability especially against temperature. In the process of immobilization, however, a decrease of enzyme activity is often observed due to the interaction of enzyme with substrate which is hampered by the insoluble immobile matrix. Hence, the selection of suitable immobile substance is very important, so that the increase of enzyme stability can be achieved, while the loss of its activity can be suppressed as much as possible. Diethylaminoethyl cellulose (DEAE-Cellulose) is one of the reagents that is frequently used in the enzyme purification process, as the use of this compound is able to increase the enzyme stability, while at the same time it minimizes the loss of enzyme activity. Previous investigation using immobilization process and chemical modification have successfully shown an increase in the enzyme stability toward pH and temperature.3-8 Based on these facts, in this research the immobilization process was chosen in an attempt to increase the stability of α-amylase which was produced, isolated, and purified from local bacteria isolate B. subtilis ITBCCB148.
Experimental
Materials
All chemicals used were of high grade (pro analysis) materials. Local bacteria isolate B. subtilis ITBCCB148 was obtained from the Microbiology and Fermentation Technology Laboratory, Chemical Engineering Department, Bandung Institute of Technology, Bandung, Indonesia.
The production, isolation and purification of α-amylase was done based on the procedure describe previously.9
Activity Test of A-Amylase and Determination of Protein Content
The activity of α-amylase was performed by iodine method10 and by the use of dinitrosalicylic acid reagent.11 The content of protein was performed by the method described in Lowry et al.12
Immobilization of Purified Enzyme with DEAE-Cellulose13
To determine the pH binding, a certain amount of ready stock DEAE-Cellulose was transferred then stabilized at various pH is using buffer of tris HCl 0.1 M with pH variation of 6; 6.5; 7.0; 7.5; 8; 8.5; 9.0; and 9.5. 0.5 mL enzyme was then added to each of the solution prepared and eluted with the suitable buffer for each pH and mixed for 5-10 minutes. The mixture was then left aside to precipitate out the DEAE-Cellulose and then decanted. The activity test was taken on the supernatant, and the protein content was also determined. To know at what pH the purified enzyme was bound, it was eluted at eluting the bound enzyme by varying pH’s and based on the ionic strength.
The Characterization of Enzyme Before and After the Immobilization Process
The characterizations of enzyme before and after the immobilization process were determined using the following methods:
Determination Of Optimum Temperature
Determination of optimum temperature of enzyme before and after immobilization was carried out by varying temperature to 55, 60, 65, 70, 75 and 80oC.
Determination of KM and Vmax. Values
The Michaelis-Menten (Km) and maximum rate reaction (Vmax) of the enzyme before and after immobilization was performed by varying substrate (amylum solution) concentration at 0.1; 0.2; 0.4; 0.6; 0.8; 1.0; 1.25 and 1.5%.
The Thermal Stability Test14
The thermal stability test was performed by measuring the residual activity of the enzyme after it has been incubated for 0, 10, 20, 30, 40, 50, and 60 minutes at the optimum temperature. The initial activity of the enzyme (without the heating process) was given the value of 100%.
Determination of Half-Life, ki and ΔGi
Determination of ki value (constant value of thermal inactivation) of purified and immobilized enzyme was done using the equation of first order inactivation kinetic as in Eq. (1).15
ln(Ei / E0 ) = -ki t …(1)
The energy change due to denaturation ( ΔGi) was done using Eq. (2).15
ΔGi = -RT ln(kih/kBT) …(2)
Results and Discussion
Determination of Optimum Temperature of Enzyme Before and After Immobilization
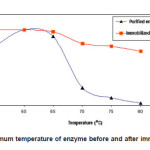
% activity of enzyme before and after immobilization at various pH’s is shown in Fig. 1. The optimum temperature of the purified and immobilized enzyme was found to be the same, which is 60°C. Similar previous studies also reported that the chemical modification and immobilization process did not always cause a change in the optimum temperature.4,15,16 Although the optimum temperature did not change, however, the stability of the enzyme has increased due to the immobilization process, especially at higher temperatures, 70-80oC. The activity of purified enzyme at 65oC was 91.1%, while the immobilized enzyme has activity of 97.4%. At higher temperature, 75 and 80oC, the immobilized enzyme has activity of 78.4 and 71.7%, respectively, while the purified enzyme has activity of only 9.9 and 2. 8%, respectively. This is because the immobilization process has caused the rigidity of the immobilized enzyme to increase; as a result it was more tolerant of temperature.
Figure 1: Optimum temperature of enzyme before and after immobilization
Determination Of Kinetic Data
The graphs of determination of KM and Vmax values are shown in Figures 2 and 3.
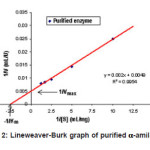
Figure 2: Lineweaver-Burk graph of purified α-amilase
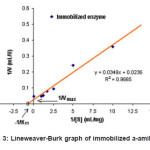
Figure 3: Lineweaver-Burk graph of immobilized a-amilase
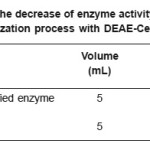
Based on the Lineweaver-Burk graph in Figure 2, the Vmax value of purified enzyme was 204 mmol mL-1 min.-1 and the KM value was 4.08 mg mL-1 substrate, whereas the immobilized enzyme has Vmax and KM values of 42.4 mmol mL-1 min-1, and 14.8 mg mL-1 substrate, respectively (Figure 3). These results indicated that the Vmax value of purified enzyme was very much different to that of the immobilized enzyme. The sharp decrease of the Vmax value in the immobilized enzyme was due to the steric hindrance of the insoluble immobile matrix, so the substrate interaction was hindered. The KM value of purified enzyme also showed discernible value to that of the immobilized enzyme. This is because the effect of the immobile substance caused the immobilized enzyme affinity toward substrate to decrease, so the activity was also sharply decreased. The decrease of enzyme activity before and after the immobilization process is presented in Table 1.
Table 1: The decrease of enzyme activity by the immobilization process with DEAE-Celullosa
The great decrease of enzyme activity in the immobilization process was similar to data previously reported where â-amylase, which was modified by silica, was decreased by 57%.4 Gaertner and Puigserver have also reported similar observation on the enzyme activity of trypsin which was modified by PEG, where the immobilized trypsin was decreased 10 times compared to the non modified one.17 This effect also caused the decrease of autolysis rate for the modified enzyme, as a result the enzyme stability was also increased.
The Enzymatic Conversion of Amylum to Glucose Using the Immobilized Enzyme with Repeat Use
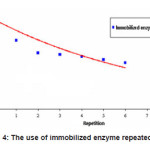
The enzymatic conversion of amylum to glucose using immobilized enzyme is shown in Figure 4.
Figure 4: The use of immobilized enzyme repeatedly
From Figure 4 it is clear that the immobilized enzyme still has high activity even after it has been used 6 times. Its activity was approximately 51.9%. This result illustrated that the use of immobilized enzyme was still effective for enzymatic conversion of amylum to glucose, although in the process of immobilization, there was activity loss of about 52.6%.
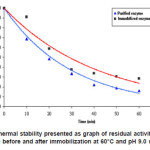
Thermal stability of the enzyme The residual activities of the purified and immobilized enzymes were determined by incubating each enzyme for 60 minutes at 60°C. At certain interval times, the activity of each enzyme was determined. The graph of thermal stability tests of the enzymes is shown in Figure 5.
Figure 5: Thermal stability presented as graph of residual activity (%) of enzyme before and after immobilization at 60°C and pH 9.0 vs time
Figure 5 showed residual activity of each enzyme at 60°C and pH 9.0 for 60 minutes. The purified enzyme has residual activity of 16.1 %, whereas the immobilized enzyme has 28.6%. This data informed us that the immobilized enzyme has thermal stability higher than that of the purified one.
The Constant of Thermal Inactivation Rate (ki), Half-Life (t1/2), and Echange Due to Denaturation
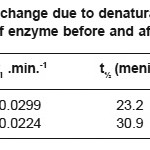
The values of constant of thermal inactivation rate (ki), half-life (t1/2), and energy change due to denaturation of purified and immobilized enzymes are presented in Table 2.
Table 2: The energy change due to denaturation (ΔGi), half-life (t½), and ki values of enzyme before and after immobilization
The half-life of immobilized enzyme was increased 1.5 times higher compare to the purified one, while the activity recovery was 47.7%. Stahl reported that thermal stability of enzyme was determined by finding the half-life of the enzyme.18 The increase of half life of the immobilized enzyme used in this research was about the same as with other data reported by others where the half-life of the enzyme was increased from 12 hours to 25 hours, while the enzyme activity recovery was 21%19. The modified enzyme with modification degree of 68% was able to increase the half life of the enzyme to 60 hours and activity recovery of 13%. The result showed that the immobilized enzyme was able to increase the half life of the enzyme similar to other data18, however, the activity loss of the immobilized enzyme with DEAE-Cellulose was much better.
The ΔGi of the immobilized enzyme was increased compared to that of the purified one. The low Gi value indicated that the enzyme was more flexible in solution which caused the denaturation of the enzyme which will occur more easily and have much higher activity, and vice versa.
It can be summarized that the immobilization process of α-amylase obtained from locale bacteria isolate B. subtilis ITBCCB148 with diethylaminoethyl Cellulose (DEAE-Cellulose) was successfully able to increase the stability of the enzyme at higher temperature, although the thermal stability was only increased 1.5 times. The repeat use of immobilized enzyme was also by far better than that of the purified enzyme. Attempts to increase stability of the enzyme used in this research will also be done with other immobile substances such as carboxy methy celullose (CMC) or local immobile substances such as bentonite and zeolite, two local clay minerals, in Indonesia.
Acknowledgments
The authors would like to thank The Directorate of Research and Community Services, Directorate General of Higher Education, The Ministry of National Education of Republic of Indonesia that provided funds for this project to be undertaken through the Strategic Research Grant Scheme (Penelitian Hibah Strategis) 2009 with contract number 1896/H26/KU/2009, 1 July 2009.
References
- Vieille C. and Zeikus J.G., Tibtech, 14: 183-189 (1996).
CrossRef
- Mozhaev V.V. and Martinek K., Enzyme Microb. Technol., 6: 50-59 (1984).
CrossRef
- Cordt S.D., Vanhoof K., Hu J., Maesmans G., Hendrickx M. and Tobback P., Biotech. Bioeng., 40: 396-402 (1992).
CrossRef
- Germain P. and Crichton R.R., J. Chem. Tech. Biotechnol., 41: 297-315 (1988).
CrossRef
- Skerker P.S. and Clark D.S., Biotechnol. Bioeng., 33: 62-71 (1989).
CrossRef
- Steadman B.L., Thompson K.C. and Middaugh C.R., Biotechnol. Bioeng., 40: 8-15 (1992).
CrossRef
- Yandri, Herasari D., Suhartati T. and Hadi S., Eur. J. Sci. Res., 23: 177-186 (2008).
- Abdel-Naby M.A., Hasehm A.M., Esawy M.A. and Abdel-Fattah A.F., Microbial. Res., 153: 319 (1998)
CrossRef
- Yandri, Suhartati T. and Hadi S., Eur. J. Sci. Res., 39: 64-74 (2010).
- Fuwa H., J. Biochem. (Tokyo), 41: 583-603 (1954).
CrossRef
- Mandels M., Raymond A. and Charles R.,Biotech. Bioeng. Symp., 6: 21-33 (1976).
- Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J., J, Biol, Chem., 193-265 (1951).
- Bollag D.M., Rozycki M.D. and Edelstein S.J.,Protein Methods 2 nd ed., John Wiley & Sons, Inc., Publication, New York, 91-93; 232-274 (1996).
- Yang Z., Michael D., Robert A., Fang X.Y. and Alan J.R., Enzyme Microb. Technol., 18: 82-89 (1996).
CrossRef
- Kazan D., Ertan H. and Erarslan A., Appl. Microbiol. Biotechnol., 48: 191-197 (1997).
CrossRef
- Francis G.E., Delgado C. and Fisher D., PEG-modified proteins in Stability of Protein Pharmaceuticals Part B (Ahern T J & Manning M C, Eds), Plenum Press, New York, 246-247 (1992).
- Gaertner H.F. and Puigserver A.J., Enzyme Microb. Technol., 14: 150-155 (1992).
CrossRef
- Stahl S., In: Thermostability of Enzymes (Gupta M N ed), Springer Verlag, New Delhi, 59-60 (1999).
- Hernaiz M.J., Montero J.M.S. and Sinisterra J.V., Enzyme Microb Technol 24: 181-190 (1999).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal