Arvind Lal1*, A. K. Gupta2, A. Kumar3 and N. K. Yadav Indu4
1Indian Railways Institute of Mechanical & Electrical Engineering IRIMEE, Jamalpur, Bihar - 811214, India.
2University Department of Chemistry, L.N.M.University, Darbhanga, India.
3Deptment of Machinical Engineering, BIT, Mesra, Ranchi, India.
4P.G. Department of Chemistry, T.M. Bhagalpur University, Bhagalpur, India.
DOI : http://dx.doi.org/10.13005/msri/070118
Article Publishing History
Article Received on : 15 Mar 2010
Article Accepted on : 17 Apr 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Bio-diesel is one of the pollution free, renewable sources of energy, providing an opportunity of employment to a large number of farmers and laboures through plantation of Bio-diesel plants like Jatropha, Karanja, Mahua, Neem, Polong etc. on a bigger scale in vast vacant land including the rail track and roadsides. The manufacturing of bio-diesel through transesterification of non-edible vegetable oils like Karanja (Pongamia Pinnata) proved to be compatible with HSD oil. The properties of Bio-diesel were found to be comparable to HSD oil by the investigation of certain physic chemical properties as per IS:1460/2000 and conforming the Bio-diesel standard as per ASTM D - 6751.
KEYWORDS:
Non-edible oil; Transesterification; HSD (High speed Diesel) oil; FFA (Free Fatty acid).
Copy the following to cite this article:
Lal A, Gupta A. K, Kumar A, Indu N. K. Y. Manufacture and Study of Physico-Chemical Properties of Karanja Bio-Diesel. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Lal A, Gupta A. K, Kumar A, Indu N. K. Y. Manufacture and Study of Physico-Chemical Properties of Karanja Bio-Diesel. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2258
|
Introduction
Global oil supplies are reaching a point where demand may outstrip supply. When this occurs, it is expected that oil prices will increase significantly. In addition, combustion of fossil oil and derivatives such as diesel and petrol releases greenhouse gases that cause global warming. Bio-diesel is seen as one alternative that can help to reduce the sustainability impacts of these events.1-2
Bio-diesel is an eco-friendly, alternative diesel fuel prepared from domestic renewable resources i.e. vegetable (edible or non-edible oil) and animal fats. These natural oils and fats are made mainly of triglycerides. These triglycerides by a chemical reaction called transesterification3-7 produce Bio-diesel.
As India is deficient in edible oils, non-edible oil may be material of choice for the production of Bio-diesel. Jatropha curcas and Karanja have been identified as the most suitable tree born oils for the production of Bio-diesel both in view of the non-edible oils available from it and its presence throughout the country. Presently, in some Indian villages, farmer are extracting oils from these seeds and after setting and decanting it they are mixing the filtered oil with diesel fuel. The fact remains that this, oil needs to be converted to Bio-diesel through a chemical reaction, trans-esterification. Large plants are useful for the centralized production of Bio-diesel.
In the present work Bio-diesel has been manufactured in small scale through transesterification of Karnja oil. It is observed that this is affected by the mode of reaction, Molar ratio of glycerides to alcohol (Methanol), type of quality of the castalyst KOH/NaOH/H2SO4, reaction temperature, washing procedure and purity of the oil taken. Physico-chemical study of Bio-disesel and Petro-diesel as per specification viz fuel composition, kinematic viscosity, flash point etc. has been carried out to observe the campatabitlity of Bio-diesel with HSD oil.
Material and Methods
Materials
Raw Vegetable oil ( Taken Karanja oil from DEE CEE MARKETING, Ranglal Jalan Road, Upper Bazar , Ranchi -834001( Jharkhand) for the experiment)
Alcohol ( Taken Methanol as experiment for better result )
Potassium /sodium hydroxide as catalyst.
Material for Titration
Iso- propyl alcohol or spirit 99% pure
Distilled water.
Phenolphthalein as indicator.
Material for Washing
Distilled water or raw water.
Bubbling processes may be also used.
( For better result distilled water used)
Method for Biodiesel Production
There are three basic routes to ester production.
Base catalysed transesterification of the oil with alcohol.
Direct acid catalysed esterification of the oil with methanol.
Conversion of oil to fatty acids, and then to alkyl ester catalysed by acid
The most commonly used method is catalysis alkali because it is highly economic for several reasons as follows.
Low temperature (upto 700C) and pressure (20 psi) processing.
High conversion (98%) with minimum side reactions and reaction time.
Direct conversion to methyl ester with no intermediate steps.
Exotic materials for construction are not necessary.
This is a pilot plant of capacity 5(five) litre made of glass having three separator, reaction vessel, alcohol recovery & auto temperature controlling device. More than 20 batches of Bio-diesel (3.5 Litre) has been made here and this bio-diesel is used in IRIMEE generator.
Bio-Diesel Reaction
The chemical reaction for the base catalysed Bio-diesel production is given below.
Once the reaction is complete after adding Potassium methoxide (30 minutes) as in case of Karanja oil, two major products are separated, Bio-diesel on the top and glycerol at the bottom. The glycerol is simply drained off from the bottom of the settling vessel.
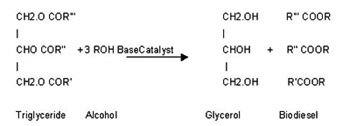
Where R’, R’’ and R’’’ are different types of fatty acid chains associated with the oil which are largely palmitic, stearic, oleic and linoleic acids ( from 16 to 18 carbon chain). For shifting the equilibrium to the right side the molar concentration ratios of alcohol: oil is kept more than 3:1. Bio-diesel is produced from vegetable oils by converting the triglyceride oils to methyl( Or ethyl) esters through a process know as transesterification. In this process the oil react with alcohol to release three ester chains from the glycerin backbone of each triglyceride. The reaction requires heat and a strong catalyst (KOH) to achieve complete conversion of the vegetable oil into the separated ester and the glycerol. The glycerol can be further purified for sale to the pharmaceutical and cosmetic industries, The mono-alkyl esters become the Bio-diesel with the lower viscosity than that of the original vegetable oil.
Process Variables in Trans-Esterification
The most important variables that influence the transesterification reaction are:
Conditioning of vegetable oils.( based on water content, sediment, total FFA etc)
Oil temperature.
Reaction temperature.
Ratio of alcohol to oil.
Types of catalyst and concentration. 6. Intensity of mixing (rpm).
Purity of reactants.
Glycerol neutralization.
Methyl ester wash.
Removal of water.
Conditioning of the Oil
It is required before the processing. It must be free from water and sediments. If the total content of FFA is more than 5 heating of the oils is required for easy processing.
Oil Temperature
The temperature at which the oil is heated before mixing with catalyst and methanol, affects the reaction. It was observed that in the case of karanja oil (Pungam), increase in oil temperature marginally increases the percentage recovery of Bio-diesel. However, the test was conducted upto 65 to 70° C, because a higher temperature results in loss of methanol.
Reaction Temperature
The rate of reaction is influenced by the reaction temperature as per the kinetics of reaction. Generally, the reaction is conducted close to the boiling point of the methanol at the atmospheric pressure. The maximum yield of esters was observed at temperature ranging from 65 to 75° C. Further increase in temperature has a negative effect on the conversion. Studies have also indicated that sufficient time and temperature are important parameters for better results.
Ratio of Alcohol to Oil
Higher molar ratio of alcohol to vegetable oil interferes in the separation of glycerol. On the other hand, with lower molar ratio, more reaction time is required for conversion but recovery decreases. It was also found that optimum molar ratio depends upon type & quality of oils.
Catalyst Type and Concentration
Alkali metal alkoxides (Methoxide) are the most effective transesterification catalyst compared to the others. The most suitable commercialised trans-esterification is conducted by KOH for better results. In case of Karanja oil upto 4 FFA, the quantity required for 3.5litre oil is 38 to 42 grams. Further increase in catalyst concentration does not increase the conversion and it adds to the extra cost because it is necessary to remove it from the reaction medium at the end. It is observed that higher amount of catalyst is required for higher FFA oils .
Mixing Intensity
The mixing effect is most significant during the slow rate region of the reaction. It is the most valuable tool for the transesterification process, while studying the kinetics of reaction. It was observed that after adding the methoxide solution to the oil, 15 to 30 minutes of stirring (upto 600 rpm in the case of pumgum oil) helps in higher rate of conversion and a better recovery upto 90% could be achieved in the lab.
Purity of Reactants
Impurities present in the oils also affect the conversion level. The quality of oils is very important in this regard. The oil settled at the bottom during prolonged storage may give lesser bio-diesel recovery because of the accumulation of the impurities like wax , higher FFA etc. But it is observed that under the condition of high temperature and pressure this problem can be overcome.
Glycerol Neutralization
The glycerol byproduct contains unused catalyst and soap that needs to be neutralised. In some cases, the salt formed after the phase separation is used as fertilizer as in the case of KOH. But in most cases the salt is left in the glycerol. For better performance it should be removed from the Bio-diesel ,other wise it will damage the engine performance.
Methyl-Ester Wash
Once separated from the glycerol, the bio-diesel is purified by washing one or more times to remove the unreacted oil, catalyst and other things like soap. For better physical property like less carbon residue it should be washed by distilled water. It is a clear amber-yellow liquid with a viscosity comparable to petro- diesel, but containing even some moisture. The soapy water or rinsed water of methyl ester is also very useful for the cleaning purposes.
Removal of Water
After washing the Bio-diesel, it contains even some water, which must be removed by simply heating upto 100 to 110° C.
Experimental Determination of Physicochemical Parameters
Specific gravity of sample of Karanja oil and Karanja Bio-diesel were determined using Hydrometers of different ranges as per ASTMD-445. Viscosity8,12 was measured by the use of Kinematic Viscometer placed in oil bath thermostated at 40 0 C. pH8 of the samples was measured by pH meter (E I, model 101). Flash point8 was measured as per ASTMD-93 by Penskey Marten closed cup apparatus. Water content and sediments (% vol) were measured by Crackling method as per ASTMD-2709. Acid value was ascertained by titration method using N/10 NaOH. Carbon residue of samples was measured by Ram’s bottom apparatus. Ultrasonic velocity9-11 in Bio-diesel was measured using ultrasonic Interferometer (Model F-81, Mittal Enterprises N. Delhi).Bulk modulus (β)13 had been calculated using the following formula
β =u2p
where u and p are ultrasonic velocity and density of Bio- diesel.
Refractive index was measured using Abbe’s Refractometer (Mittal Enterprises N. Delhi)
Results and Discussion
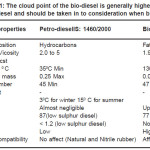
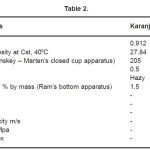
The physical properties of bio-diesel are compared to the petro-diesel in Table 1 The bio-diesel sample was tested as per IS: 1460/2000 and ASTMD-6751 in the Diesel-shed Laboratory at Jamalpur Bihar. This specification deals with testing of HSD oil which is the middle distillate of the petroleum refining process. Experimental values of physicochemical parameters have been given in Table 2.
Table 1: The cloud point of the bio-diesel is generally higher than petro-diesel and should be taken in to consideration when blending
Table 2
Figure 1: Bio-Diesel reactor
The viscosity of liquid is an important characteristic as it determine the ease of flow through pipeline, injector nozzle and orifices and formation of fuel in the cylinder. The viscosity of karanja oil was found to be higher compared to the Karanja oil ester and to be within the prescribed limit as per ASTM D-6751 as mentioned in Table 1 & 2. Flash point measures the tendency of the sample to form a flammability mixture with air. It was found to be higher in comparison to the HSD oil and found to be within limit. The acid value can be used as a guide in quality control of fuels. In the case of Karanj oil it is found to be below 5. Similarly, higher the carbon residue value, the greater will be carbon deposits in the combustion chamber. Therefore, carbon residue is intended to provide some indication to the relative choking properties. It was observed that the carbon residue of Karanj oil was higher than the Karanj Bio-diesel as per ASTMD 7651. The Refractive Index in this case was found to be 1.475. Ultrasonic velocity has been measured to calculate the Bulk modulus of compressibility. The latter is useful in the study of its effects on fuel injection timing of Bio-diesel. The Ultrasonic velocity in this case has been found to be 1404m/s, which is higher than the HSD oil ( 1358m/s). It is a clear indication of its higher fuel injection timing. The measurement of the bulk modulii of blends of Bio-diesel with HSD oil of different composition to understand its interaction with fuel injection timing and certain other fuel properties.
Acknowledgements
The authors would like to acknowledge the Director, IRIMEE to provide the research facilities at IRIMEE ,Jamalpur.
References
- Sharma M.P. and Barnwal B.K.(2005), “Prospects of Biodiesel Production from vegetable oils in India”. Renewable and sustainable Energy Reviews, 9: 363-378. (2005)
CrossRef
- Lal,A.,chowdhury,G.,Kumar,A.,and Yadav,N.K., Prospects of Renewable Energy In Indian Railways A-Challenge Proceedings of the International Conference on renable energy Asia 129- 135 (2008).
- Otera J. Chem. Rev. 93: 1449 (1993).
- Wright H.J., Se gur, J.B., clark. H.V., coburn, S.K., Langdon E.E., and Dupuis, E.N. oil and soap. 145. (1944)
- Freedman B., Butterfield, R.O. and pryde, E.H.J. Am. Oil chem.. Soc. 63: 1375. (1986)
CrossRef
- Chandraju and Pratima,B.K. Asian J. Chem, 16(3-4): 1811-1818. (2004).,
- Canakci, M., Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Am Soc Agric Eng., 4: 1429-1436. (2001).
- R.D.S.O/ LKO/ REPORT NO.MP.Misc150,Page 4, Talele 2, ( 2003)
- Yadav R.B., Gupta A.K. and Yadav C.K., J. Ind. Council. Chem Vol. XIV, No. 2,(1997)
- Gupta A.K. , Yadav C.K.and Kumar Krihshna, J. Ind. Council. Chem 21(2): 32 (2004)
- Gupta A.K. Kumar Krihshna, and Karn Birendra Kumar, J. Ind. Council. Chem 26(1): 77-81 (2009)
- Gupta A.K. and Kumar Krihshna, J. Ind. Council. Chem . 24(2): 46-50 (2007)
- Boehman Andre.,Alam Mahabubul., SongJuhun., Acharya Ragini and miller Kirk, Proceedings of DOE Diesel engine Emissions Reduction conference August 2428, Newport, RI (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal