Study on the Performance of Ajowan Seeds Extract on the Corrosion of Steel in Acidic Solution by Effect of Temperature
Aisha M. Al-Turkestani
King Abd El-Aziz University, Girls College of Education, Chemistry Department, Jeddah, KSA
DOI : http://dx.doi.org/10.13005/msri/070116
Article Publishing History
Article Received on : 05 May 2010
Article Accepted on : 07 Jun 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Electrochemical measurements (EIS and PDP) have been used to study the temperature effect in the range (30p C - 70p C) on the performance of Ajowan seeds extract (ASE) and its inhibitive effects on the corrosion of steel in 2.0M H2SO4. A good agreement between the data obtained by impedance and polarization measurements was found. Results obtained reveal that ASE reduces the corrosion rate at all temperatures studied. The inhibition efficiency increases with rise temperature and the activation corrosion energy decrease in presence of ASE suggest a chemical adsorption model for the corrosion processes.Thermodynamic parameters, Ea, ΔH#, ΔS# have been calculated and are discussed.
KEYWORDS:
Steel; Acidic inhibition; Temperatur effect; Acidic corrosion
Copy the following to cite this article:
Al-Turkestani A. M. Study on the Performance of Ajowan Seeds Extract on the Corrosion of Steel in Acidic Solution by Effect of Temperature. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Al-Turkestani A. M. Study on the Performance of Ajowan Seeds Extract on the Corrosion of Steel in Acidic Solution by Effect of Temperature. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2252
|
Introduction
The inhibition of corrosion for steel is the subject of tremendous technological importance due to the increased industrial applications of these material. Several authers (1-4) have studied the corrosion of steel and its inhibition by organic and natural inhibitors in acid solutions.
Inhibition are widely used for protection of materials from corrosion in acid environments. Usually, inhibitors protect the metal by adsorbing onto the surface and retard metal corrosion in aggressive media, so selecting the appropriate inhibitor for metal is very important. Furthermore, in order to improve the performance of inhibitor and reduce the corrosion of metal in acidic media, the temperature effect are widely used in case of acidic corrosion, mainly in hydrochloric and sulphuric acids.1-11
The study of the mechamism of corrosion inhibitors action has a great importance both for the formulation of new inhibitors and for the correct use of these inhibitors under different conditions. In many industrial operations, like pickling, acid cleaning, acid descaling and oil well acidizing, the choice of optimum working temperature and process duration is of particular importance. The inhibitors used are expected to be chemically stable and to provide high protective effect under the condition discussed.
The temperatures has a great effect of the rate of metal electrochemical corrosion. In case of corrosion in acid medium (hydrogen depolarization), the corrosion rate increases exponentially with temperature increase because the hydrogen evolution over-potential decreases.12 An experimental dependence of Arrhenius type is observed between the corrosion rate and temperature.
The purpose of this study is the effect of temperature on steel corrosion in presence of Ajowan seeds extract (ASE) to improve the performance of ASE as safe inhibitor.
Experimental
The steel used had the following chemical composition (wt%): 0.38% C, 0.15% Mo, 0.50% Mn, 0.40% Si, 0.03% S, 0.035% P and 97.61% Fe.
Tow electrochemical measurements: impedance spectroscopy (EIS) and ptentiodaynamic polarization (PDP) were used to determine the corrosion inhibition characteristics of ASE.
Electrochemical measurements are carried out in a conventional three electrodes electrolysis cylindrical pyrex glass. A steel cylinder pressed into a Taflon holder acted as a working electrode (WE). Its working area of 0.791cm2 remained precisely fixed. A saturated silver/silver chloride electrode connected through a salt bridge was used as reference electrode (RE), while a large area platinum leaf was used as counter electrode (CE). The measurements were made by connecting the electrochemical cell to ACM Gill AC and to a Samsung computer (Bridge DVD ASUS 8X max. Impedance data were obtained in the frequency range 0.5Hz- 30KHz and potentiodyanamic polarization curves were recorded at scan rate between -200mV -700mV.
Prior to each electrochemical experiment the WE was wet polished with 60 to 1200 grade emery paper, riesed with bi-distilled water and acetone, then immediately inserted into the glass cell, that contained 250 ml of electrolyte solution. Hence, all the electrochemical experiments were carried out after 20 mints. of exposure to the solution.
Results and Discussion
Temperature has a great influence on the rate of steel corrosion in acidic medium. An increase in temperature results in an exponential increase in corrosion rate due to decrease in hydrogen evolution overpotential (11,13).
To gain insight into the nature of inhibitor adsorption, the effect of temperature in the range from 30° C to 70° C on the corrosion behavior of steel in absence and presence of fixed concentration of ASE was investigated using electrochemical technique (impedance (Figs. (1 and 3)) and polarization (Figs. (2 and 4)). The results obtained indicate that the rates of steel corrosion in absence and presence of ASE increased with rise in temperature and the ASE molecule was adsorbed on the steel surface at all temperatures studied.
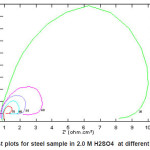
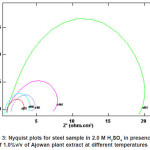
Figs. (1 and 3) show the Nyquist plots of steel in 2.0M H2SO4 in absence and presence of ASE at 30, 40, 50, 60 and 70p C, respectively. The complex impedance plots have the appearance of adepressed semicircle with centers below the Z‘-axis for all solution examined in Fig. 1, the plots refer to non-ideal capacitive semicircle. Acharacteristic feature of the results is the absence of a diffusive contribution to Z‘ at low frequencies. This indicates that the charge trancfer control the dissolution mechanism of steel across the phase boundary in the absence of the inhibitor. The results also indicate that as the temperature increases, the rate of steel corrosion increases. Similar Figure (3) in presence of 5%v/v of ASE inhibitor at different temperatures. The results indicate that rising up the temperature leads to a decrease in the charge transfer resistance, hence an increase of the corrosion rate of steel is observed. Similar behavior are obtained by E. Khamis et al.14
Figure 1: Nyquist plots for steel sample in 2.0 M H2SO4 at different temperatures
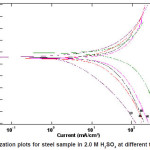
Figure 2: Polarization plots for steel sample in 2.0 M H2SO4 at different temperatures
Figure 3: Nyquist plots for steel sample in 2.0 M H2SO4 in presence of 1.0%v/v of Ajowan plant extract at different temperatures
Figure 4: Polarization plots for steel sample in 2.0 M H2SO4 in presence of 1.0%v/v of Ajowan plant extract at different temperatures
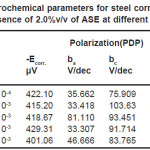
It obvious from Figs. (2) and 4) that with increasing temperature, the anodic and cathodic current densities Icorr. also increase, indicating that the rate of the corresponding electrode processes (metal dissolution and hydrogen evolution) increases, but the Ecorr. for steel increased more rapidly with temperature in the absence of inhibitor. These results confirmed that ASE acts as an efficient inhibitor in the range of temperature studied. The corresponding data are shown in Tables (1&2).
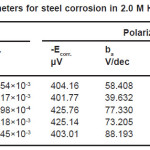
Table 1: Electrochemical parameters for steel corrosion in 2.0 M H2SO4 at different temperatures
Table 2: Electrochemical parameters for steel corrosion in 2.0 M H2SO4 in presence of 2.0%v/v of ASE at different temperatures
It could be seen, from Tables (1&2) that the rise in temperature lead to an increase in corrosive rate without and with inhibitor. It was also noted that the inhibition efficiency of Ajowan extract increased with rise in temperature. This may be attributed to chemical adsorption of Ajowan extract molecules on steel surface and it can be explained by the specific interactions between the steel surface and the inhibitor.15-17
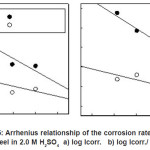
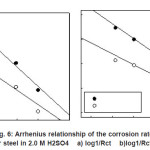
Arrhenius plots for the charge transfer resistance (Rct) and/or the corrosion current densities (Icorr.) of steel in absence and presence of ASE are given in Figures (5-6). The activation energies from EIS and PDP methods can be calculated from the following relationships:
1/Rct and/or Icorr = K exp (-Ea / RT) …(1)
log ( 1/Rct) and/or log Icorr = log K – Ea/RT …(2)
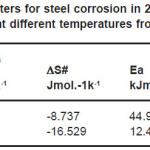
where 1/Rct and/or Icorr represents the corrosion rate, K is constant, R is the universal gas constant and T is temperature in Kelvin.The values of Ea from EIS and PDP measurements were recorded in Table 3.
Figure 5: Arrhenius relationship of the corrosion rates for steel in 2.0 M H2SO4 a) log Icorr. b) log Icorr./ T.
Figure 6: Arrhenius relationship of the corrosion rates for steel in 2.0 M H2SO4 a) log1/Rct b)log1/Rct/T
Table 3: Activation parameters for steel corrosion in 2.0 M H2SO4 in absence and presence of 2.0%v/v of ASE at different temperatures from EIS and PDP measurements
It obvious that Ea values from EIS and PDP methods in presence of ASE were lower than that in their absence, respectively, this means that an inhibitor becomes more effective as the temperature increases18 which it can explained as due to each of the following:
An increase in surface area of the metal covered by inhibitor molecules as the temperature rises.19
The chemisorptions of Ajowan inhibitor on steel surface.11,20-23
Such inhibitor ASE, like the catalysts which do not change the temperature coefficient of the reaction are firmly held on the metal surface.19
Machu24 suggest that, the kinetic of such corrosion process acquires the character of a diffusion process, in which, at high temperatures the amount of inhibitor present at the metal surface is greater than that at lower temperatures. This increase the surface area of the metal covered by the inhibitor molecules. Then, the reaction between the metal and the inhibitor can then only take place by diffusion of acid anions through the pores of the protective layer formed.
Hoar and Holliday26 suggest that the enhancement of inhibitive efficiency at higher temperatures may be due to the higher of activation energy available for adsorption, and the higher rate of diffusion of inhibitor molecules.
Singh et al.27, consider that with increase in temperature some chemical changes occure in the inhibitor molecules leading to an increase in the electron densities at the adsorption centers of the molecule causing an improvement in inhibitor efficiency.
For calculating the enthalpy ΔH# and entropy ΔS# of activation, the alternative formulation of the Arrhenius equation is the transition state equation28-30:
(1/Rct or Icorr.)= RT/Nh exp(ΔS#/R) exp (-ΔH#/RT) …(3)
log(1/Rct/T) = log(R/Nh)+ ΔS#/2.303R- ΔH#/2.303RT …(4a)
log(Icorr./T) = log(R/Nh)+ ΔS#/2.303R- ΔH#/2.303RT … (4b)
being h the blank’s constant and N Avogadro’s number. Figs. (5 &6) show straight lines of plots of logRct/T and/or logIcorr/T vs. 1/T with a slope of ΔH#/R and intercept of log(R/Nh)+ ΔS#/R. Values of Ea, ΔH# and ÄS# are collected in Table(3). The data show that the thermodynamic parameters (ΔH# and ΔS#) of the dissolution reaction of steel in 2.0M H2SO4 in the presence of ASE are higher than those of the non- inhibited solution from both EIS and PDP methods. The negative values of ΔH# suggest that the dissolution process is an exothermic phenomenon and the dissolution of steel is difficult. Also, the entropy ΔS# widely decreases with the content of the inhibitor. This means the formation of an ordered stable layer of inhibitor on steel surface.23,31
Conclusion
The principl finding of the present work could be summarized as follows:
The corrosion rate of steel in 2.0M H2SO4 fromboth impedance and polarization methods is directly proportional to an increase in temperature from 20°C to 70°C.
The inhibition efficiencu of Ajowan seeds extract (ASE) increases with the temperature and the activation corrosion energy decreases in presence of inhibitor from both EIS and PDP, which indicate that the chemisorptions mode is more probable.
ASE has abetter performance for inhibit the corrosion of steel in 2.0M H2SO4 at high temperatures.
References
- I.A. Ammar, F.M. El Khoraf, Werkst. Korros. 24: 171 (1982).
- I.H. Omar, G. Trabanelli, F. Zucchi, in: Proceedings of the 10th ICMC, Madras, India, 2723 (1988).
- G. Perboni, G.Rocchini, in Procceedings of the 10th ICMC, Madras, India, 2763 (1988).
- F. Zucchi, G.Trabanelli, in: Proceedings of the Seventh European Symposium on Corrosion Inhibitors, Ferrara, Italy, 339 (1990).
- Q.J. Slaiman, H.M. Al-Saaty, in:Proceedings of the Seventh European Symposium on Corrosion Inhibitors, Ferrara, Italy, 189 (1990).
- G. Moretti, G. Quartarone, A. Tassan, A. Zingales, Electrchim. Acta 41: 1971 (1996).
CrossRef
- J. de Damborenea, J.M. Basidas, A.J. Vazques, Electrochim. Acta 42: 455 (1997).
CrossRef
- R. Hariharaputhan, A. Subramanian, A.A. Antony, P.M. Sankar, A. Gopalan, T.Vasudevan, I.S. Vancatakrishna, Brit. Corros. J. 33: 214 (1998).
CrossRef
- L. Elkadi, B. Mernari, M. Traisnell, F. Bentiss, M. Lagrenee, CORROS. Sci. 42 (2000).
- F. Bentiss, M. Traisnle and M. Lagrenee, J. App. Electrochem. 31: 41 (2001).
CrossRef
- A. Popova, E.Sokolova, S.Raicheva, M. Christov, Corros. Sci. 45: 33 (2003).
CrossRef
- E.E. Oguzie, Pigment & Resin Technology, 34(6): 321 (2005).
CrossRef
- E. Noor, Int. J. Electrochem. Sci., 2: 996 (2007).
- E. Khamis, A. Hefnawy and A.M. El-Demerdash, Mat.-wiss. U. Werkstofftech., 38(3): 227-232 (2007).
CrossRef
- I.A. Ammar,F.M.El Khorafi, Werkstsf & Korros. 24: 702 (1973).
CrossRef
- A. Bouyanzer and B. Hammouti, Pigment & Resin Technology, 33(5): 287 (2004).
CrossRef
- E. Chaieb, A. Bouyanzer, B. Hammouti and M. Benkaddour, Appl.Surf. Sci. 246: 199 (2005).
CrossRef
- S.A. Balesin and S.K. Novikov, Zh. Prikl. Khim., 24: 283 (1951).
- I.N. Putilova, S.A. Balezin and U.P. Barannik, Metallic Corrosion Inhibitors P.31, Pergamon Press, New York (1960).
- S. Sankarapapavinasam, F. Pushpanaden, M. Ahmed, Corros. Sci., 32: 193 (1991).
CrossRef
- F. Bentiss, M. Lagrenee, M. Traisnel and J.C. Hornez, Corrosion, 55(10): 968 (1999).
CrossRef
- F. Bentiss, M. Traisnel and M. Lagrenee,J.App. Electrochemistry, 31: 41 (2001).
CrossRef
- M. Bouklah and B. Hammouti, Portugalia Electrochimica Acta 24: 457 (2006).
CrossRef
- Z. Hauptmann, U. Graefe, H.Remane, Organic Chemistry, Nauka I Izkustvo, Sofia (1985).
- W. Machu, Korros. Metall., 14: 324 (1938).
- T.P. Hoar and R.D. Holliday, J. Appl. Chem., 3: 502 (1953).
CrossRef
- D.D.N. Singh, R.S. Chaudhary, B. Prakash and C.V. Agarwal, Br.Corros. J., 14: 235 (1979).
CrossRef
- I.N. Levine, Physical Chemistry, Ed. McGraw-Hill Kogakusha, Ltd, ISBN 0-07- 037418-X,768 (1978).
- A.J. Bsrd and L.R. Faulkner, Electrchemical Methods, Wiley & Sons (1997).
- P.W. Atkins, Elements de Chimie Physique, Ed. De Boeck Universite, 258 (1998).
- M.M. Osman, Anti-Corros. Meth. Mater. 45:176 (1998).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal