K. K. Dubey, V. Nayar* and P. S. Choudhary*
C.M.D.PG College Bilaspur, India.
G.B.V.P College Hardibazar, Korba, India.
DOI : http://dx.doi.org/10.13005/msri/070124
Article Publishing History
Article Received on : 02 Dec 2009
Article Accepted on : 10 Jan 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Zinc Sulfide nanoparticles were prepared by chemical rout i.e. co-precipitation method. X-ray diffraction profiles of ZnS have been conformed as single phase with hexagonal structure. And crystalline in nature. The lattice parameters of prepared material is a= 3.8314A0 c=6.2431A0 with space group P63mc. The particle size was determined by scherer formula and found to be 28 nm. The band gap energy of ZnS nanoparticles was determined by optical absorption experiment and found to be 3.68 eV at 300oK. Photoluminescence spectra ware recorded by luminescence spectrophotometer. All the plots contains two peak centered at 315 nm and 425 nm. The excitation wavelength was 250 nm. Appearance of broad peaks centered at 425 nm is attributed to the presence of sulphur vacancies in the lattice.
KEYWORDS:
Electro ceramics; Dielectrics; Microstructure; Sintering; Co-precipitation method
Copy the following to cite this article:
Dubey K. K, Nayar V, Choudhary P. S. Synthesis, Structural and Optical Properties of Zns Nano-Particles. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Dubey K. K, Nayar V, Choudhary P. S. Synthesis, Structural and Optical Properties of Zns Nano-Particles. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2277
|
Introduction
In the last few years, nano-structured materials have attracted a great deal of attention due to their unique characteristics which cannot be obtained from conventional macroscopic materials. Nano-particles can display novel optical, electronic, magnetic, chemical, and structural properties that might find many important technological applications. The properties and applications of the nano-particles are largely dependent on their size and morphology. The most evident manifestation properties are the optical light emission in the blue–red spectral region characterized by a blue shift at smaller crystallite dimensions.1 Such properties make semiconducting nanostructures suitable for several kinds of applications, from antireflecting coatings2 to bioelectronics3 and light emitting devices.4 In the past decade, semiconductor nano-particles attract much attention because of their size-dependent (and thus tunable) photo-and electro- luminescence properties and promising applications in optoelectronics. Among the family of II–VI semiconductors, ZnS [5,6., CdS [7., ZnO,8 CdTe,9 etc. are the foremost candidates because their favorable electronic and Optical properties for optoelectronic applications. Among those ZnS is a commercially important II–VI semiconductor having a wide optical band gap, rendering it very attractive material for optical application especially in nanocrystalline form. ZnS can have two different crystal structures (zinc blende and wurtzite), both of which have the same band gap energy (3.68 eV) and the direct band structure. ZnS has been used for the cathode ray tube, the field emission display, and the scintillator as one of the most frequently used phosphors.10-12 In addition, a ZnS crystal laser has been produced using streamer excitation,13 and thin films of ZnS can be used as an active emitting material in such a device, termed the hot electron cold cathode.14 Optical and luminescent properties of nanocrystalline ZnS prepared in the forms of thin film, powder and colloid using different synthesis techniques such as sputtering,15 co-evaporation,16 wet chemical,17-19 sol-gel20,21 solid state22 micro-wave irradiation,23,24 ultrasonic irradiation25 or synthesis under high-gravity environment26 were studied in detail. Luminescence measurements were identified as one of the most important techniques to reveal the energy structure and surface states of these particles.17 Localized trap states inside the band gap were studied27 in detail to recognize the sub-band gap energy levels. It was found that the defect levels play an important role in determining the luminescence characteristics of the ZnS nanoparticles.28 We, therefore, attempted to prepare pure ZnS nanopartice particle and structurally characterized. To the best of our knowledge, no such detailed report exists in literature.
Material and Methods
Semiconductor nanoparticles can have quantum effects and have high emission yield across the visible and near infrared spectrum. The surface of such nanoparticles or quantum dots largely determines the quantum yield and emission life time of the band gap luminescence. High luminescence yields are achieved by the use of surface passivation to reduce the non-radiative surface recombination of charge carriers. Two methods of passivation are commonly employed, one is through so called band gap engineering and another method is to absorb Lewis bases on to the surface. For the growth of large band gap semiconductor on the surface of nanoparticles, the growth condition must be controlled such that no homogeneous nucleation would occur, but only a growth proceeds on the surface of the nanoparticles. Therefore the concentration of the growth species needs to be controlled such that the super saturation is not high enough for nucleation, but high enough for growth. Now applied to control the super saturation of growth species by the drop wise addition of growth precursor solution in to the reaction mixture, which consists of growth nanoparticles. All chemicals were used of analytical grade. Double distilled water was used as a solvent. Zinc acetate dihidrate (Zn (CH3COOH)2.2H2O 98.9%), sodium sulphide nanohydrate (Na2S.9H2O 98.9%), sodium hexa metaphosphate (NaPO4)6 were purchased for Loba Chemicals Pvt. Ltd., Bombay. ZnS nanoparticles have been prepared by co-precipitation method in our experimental work dilute solution (1 mole concentration ) of zinc acetate and sodium sulfide (1 mole concentration )were mixed in the presence of (capping agent ) sodium hexa meta phosphate (SHMP). The aqueous solution of sodium hexa meta phosphate was added drop wise with the help of measuring burette in the rate of 1 ml per minute while stirring the solution continuously magnetic stirrer was used for stirring the solution, then the solution is heated till boiling after cooling at room temperature 1 mole solution of Na2S:9H2O was mixed drop wise with constant stirring into the solution subsequently a milky color solution was obtained and this solution was kept in oven for 24 hours then the while precipitate settles down in the bottom of the flask. This precipitate was removed and washed several times with double distilled water then capping agent and excess ions were removed by washing the solution several times and finally washes with alcohol (ethyl alcohol) for 10 minutes and this washed solution was centrifuged finally the precipitate was spread over a glass substrate and dried in an oven upto 400°C for 24 hours at the room temperature capping agent (SHMP) control the particles size for the present investigation a number of samples were prepared by using different concentration of capping agent. The size of the particle is controlled by changing pH value of the whole solution.
pH controlled the rate of reaction due to the common ion effect. At higher pH the solubility product increases and as a result no formation of ZnS nano-particle is formed.
Results and Discussion
Structural Study
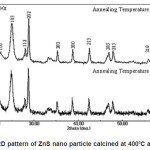
Figure 1 shows X-ray diffraction profile of ZnS. The compositions have been conformed as single phase with hexagonal structure. The lattice parameters of prepared material is a= 3.8314A0 c=6.2431A0 with space group P63 mc.
Figure 1: XRD pattern of ZnS nano particle calcined at 4000C and 6000C
The particle size was determined using Scherer formula. Accordingly
Crystalline size=K*λ/F W H M*Cosθ … (1)
Where K= 0.9, λ= 1.54056A0 for Cu Kα F W H M = Full Width at half maximum of the strongest line. The particle size was found to be 28nm.
Photo Luminescence Study
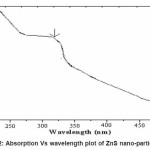
It is evident from the Figure 2 that sample exhibit a strong absorption at wavelength 298nm for ZnS suggesting blue shift with respect to the bulk arising from quantum confinement effect in the nanoparticles. The band gap of bulk ZnS is 3.68 eV at 300K.The band gap energy of the samples are calculated from the optical absorption experiment. From the absorbance (A) and film thickness (t), the absorption coefficient á (cm-1) is calculated using the equation
α = 2.3026 (A/t) …(2)
Thickness of sample is measured by Gravimetry method using the electronic balanced. According to Gravimetry method the film thickness can be calculated as
Thickness (t) = M/ηa …(3)
Where M=mass of the deposited film, η=density of ZnS and A=area of film. The band gap energy of the film at wavelength range 200-500 nm has been calculated using the equation (4)
α2 = A (hν – Eg ) …(4)
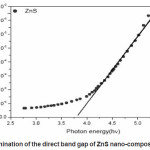
Where A is a constant related to the effective masses associated with the bands, hí is the photon energy and Eg is the band gap energy. The band gap energy is measured by a graph of á2 versus hí data using absorption spectra. The extrapolation (Fig.3) of the straight line to α2=0 give the value of band gap energy. Effective mass approximation (EMA) as proposed [29,30. has been used to explain the change of energy gap as a function of particle size. According to EMA the equation derived for radius of particle is given by
ΔEg= Eg(film)– Eg(bulk)=[h2/8μr2.-1.8e2/ε0εr ….(5)
Where 1/µ =1/me* +1/mh* is the reduced mass of electron hole effective mass, me* = 0.34m0 and mh* = 0.23m0 and εr=8.76 is the permittivity of the sample.31 The calculated particle size is 28nm for ZnS which is good agreement determined by XRD.
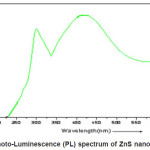
Photoluminescence spectra measured at room temperature (2900K) of the nanocrystalline ZnS is shown in the fig.(4.) These spectra were recorded using (Thermospectronic AMINCO) luminescence spectrometer. All the plots contains two peak centered at 315nm and 425nm .The excitation wavelength was 250nm. Appearance of a broad peaks centered at 425nm is attributed to the presence of sulphur vacancies in the lattice.32 This emission results from the recombination of photo generated charge carriers in shallow traps.33, 34 The other peak centered at 315nm is due to the band to band transition of nanocrystalline ZnS.
Conclusions
In the present investigation for the preparation of ZnS nano particle by co-precipitation method has been used and its structural and optical properties has been discussed briefly. High luminescence yields are achieved by the use of surface passivation to reduce the non-radiative surface recombination of charge carriers. Band gap engineering method are employed in surface passivation for the present work to control the super-saturation of growth species. We are applied by the drop wise addition to the growth precursor Solution in to the reaction mixture with consists of growth nano-particles. It is evident from absorption co-efficient (á2) versus photon energy hí that sample exhibit a strong absorption at wavelength 298nm for ZnS suggesting blue shift with respect to the bulk arising from quantum confinement effect in the nano-particles. In the photo-luminescence spectrum of ZnS nano-particles appearance of broad peaks centered at 425 nm is attributed to the presence of sulphur vacancies in the lattice and the other peak centered at 315 nm is due to the band to band transition of nano-crystalline ZnS.
Figure 2: Absorption Vs wavelength plot of ZnS nano-particles
Figure 3: Plot for the determination of the direct band gap of ZnS nano-composite of ZnS nanoparticles
Figure 4: Photo-Luminescence (PL) spectrum of ZnS nano-particles
Acknowledgements
The authors are grateful to University Grant Commission (CRO) Bhopal, for providing financial assistance without which this work could not have been possible. We also wish to thank the Principal, Chairman and Head of the Department in our college.
References
- R. Rossetti, L. Ellison, J. M. Gibson, and L.E. Brus, J. Chem. Phys. 80(9): 4464, (1984).
CrossRef
- J. H. Park, J. Y. Kim, B. D. Chin, Y. C. Kim, and O. O. Park, Nanotechnology 15: 1217(2004).
CrossRef
- E. Katz, and I. Willner, in Nanobiotechnology, Wiley-VCH (Ed.), Weinheim (2004).
CrossRef
- V. L. Colvin, M. C. Schlamp, A. P. Alivisatos, Nature 370: 354 (1994).
CrossRef
- A.D. Dinsmore, D.S. Hsu, H.F. Gray, S.B.Quadri, Y. Tian, B.R.Ratna, Appl. Phys.Lett.75: 80 (1999).
CrossRef
- R. Maity, K.K. Chattopadhyay, Nanotechnology 15: 812 (2004).
CrossRef
- J. Tittel, W. Gohde, F. Koberling, Th. Basche,A. Kornowski, H.Weller, A. Eychmuller, J.Phys. Chem. B 101: 3013 (1997).
CrossRef
- S. Mahamuni, K. Borgohain, B.S. Bendre, V.J.Leppert, S.H. Risbud, J. Appl. Phys. 85: 2861(1999).
CrossRef
- A.L. Rogach, Mater. Sci. Eng. B 69-70: 435(2000).
CrossRef
- H. Okuda, J. Dakada, Y. Iwasaki, N.Hashimoto, C.Nagao, IEEE Trans. Consumer Electron. 36: 436 (1990).
CrossRef
- J. Ghrayeb, T.W. Jackson, R. Daniels, D.G.Hopper, Proc. SPIE 3057: 237 (1997).
CrossRef
- J.C. Barton, P.W. Ranby, J. Phys. E 10: 437(1977).
- A.Z. Obidin, et al., Sov. J. Quantum Electron.18: 1764 (1988).
CrossRef
- N. Dalacu, A.H. Kitai, Appl. Phys. Lett. 58:613 (1991).
CrossRef
- S.K. Mandal, S. Chaudhuri, A.K. Pal, Thin Solid Films 30: 209 (1992).
CrossRef
- R. Thielsch, T. Böhme, H. Böttcher, Phys.Status Solidi, A 155: 157 (1996).
CrossRef
- W. Chen, Z. Wang, Z. Lin, L. Lin, J. Appl. Phys.82: 3111 (1997).
CrossRef
- S.M. Scholtz, R. Vacassy, J. Dutta, H.Hoffmann, J. Appl. Phys. 83: 7860 (1998).
CrossRef
- J. Nanda, S. Sapra, D.D. Sarma, N.Chandrasekharan, G. Hodes, Chem. Mater.12: 1018 (2000).
CrossRef
- M. Tan, W. Cai, L. Zhang, Appl. Phys. Lett.71: 3697 (1997).
CrossRef
- B. Bhattacharjee, D. Ganguli, S. Chaudhuri,A.K. Pal, Mater. Chem. Phys. 78: 372(2003)
CrossRef
- H.-Y. Lu, S.-Y. Chu, S.-S. Tan, J. Cryst. Growth 269: 385 (2004).
CrossRef
- Y. Zhao, J.-M. Hong, J.-J. Zhu, J. Cryst. Growth 270: 438 (2004).
CrossRef
- Y. Ni, G. Yin, J. Hong, Z. Xu, Mater. Res. Bull.39, 1967 (2004).
CrossRef
- J.F. Xu,W. Ji, J.Y. Lin, S.H. Tang, Y.W. Du,Appl. Phys., A 66: 639 (1998).
CrossRef
- J. Chen, Y. Li, Y. Wong, J. Wang, J. Yun, D.Cao, Mater. Res. Bull. 39,185 (2004).
CrossRef
- D. Denzler, M. Olschewski, K. Sattler, J. Appl.Phys. 84: 2841 (1998).
CrossRef
- K. Kanemoto, H. Hosokawa, Y. Wada, K.Murakoshi, S. Yanagida, T. Sakata, H. Mori,M. Isikawa, H. Kobayashi, J. Chem. Soc.,Faraday Trans. 92: 2401 (1996).
CrossRef
- A.D.Yoffe, Adv. Phys 42: 173 (1993)
CrossRef
- Y.S.Yuang,F.Y.chen,Y.Y.Lee,C.L.Liu Jpn. J.Appl. Phys. 76: 304 (1994).
- Landolt-Bornstein. Numerical data and functional relationships in science and technology (Berlin: Springer Verlag) Vol.22a, p. 168 (1987).
- N.A.Dhas,A.Zaban,A.Gedanken,Chem.mater 11: 806 (1999).
CrossRef
- K.Sooklal,B.S.Cullum,S.M.Angel,C. J. Murphy,J.Phys.Chem.100, 4551 (1996).
CrossRef
- W.G.Becker,A.J.Bard, J.Phys.Chem., 87,4888 (1983).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal