P. Geetha1, P. Ramesh2, K. Raju3, Hema Tresa Varghese4 and C. Yohannan Panicekr5*
1Department of Physics, Govt. Arts and Science College, Calicut, India.
2Department of Physics, University of Calicut, Calicut, India.
3Department of Physics, Univeristy College, Thiruvananthapuram, India.
4Department of Physics, Fatima Mata National College, Kollam, India.
5Department of Physics, TKM College of Arts and Science, Kollam - 691 005, India.
DOI : http://dx.doi.org/10.13005/msri/070135
Article Publishing History
Article Received on : 30 Dec 2009
Article Accepted on : 01 Feb 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The thermodynamic excess functions of a composition of chloroform and ketones were studied in this work. The ketones used as acetophenone, cyclophexanone and ethyl methyl ketone. Ultrasonic velocity for the liquid mixtures are measured over a wide range of concentrations, using a single crystal ultrasonic interferometer at a frequency of 2 MHz. For binary mixtures of chloroform with acetophenone and cyclohexanone, surface tension increases with increase in concentration of the ketone. But for the binary mixtures of chloroform and ethyl methyl ketone surface tension decreases with increase in concentration of the ketone.
KEYWORDS:
Thermodynamic excess functions; Acetophenone; Binary; Ternary mixtures
Copy the following to cite this article:
Geetha P, Ramesh P, Raju K, Varghese H. T, Panicekr C. Y. Ultrasonic Studies on Binary and Ternary Mixtures of Some Organic Liquids. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Geetha P, Ramesh P, Raju K, Varghese H. T, Panicekr C. Y. Ultrasonic Studies on Binary and Ternary Mixtures of Some Organic Liquids. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2316
|
Introduction
The study of different states of matter, especially the liquid and gaseous states, statistical methods are being extensively used. A self consistent theory for liquids is not available even now. The main reason for this is the very complex nature of interactions in the liquid state. The statistical approach is to calculate the partition function and then establish the equation of state through thermodynamic variables. Ultrasonic investigation of liquid mixtures containing of polar and non-polar compounds is of considerable importance in understanding intermolecular interaction between the component molecules and they find applications in several industrial and technological processes.1,2 Further such studies as a function of concentration are useful in gaining insight into the structure and bonding of associated molecular complex and other molecular processes. The ultrasonic velocity measurements finds wide applications in characterizing the physico-chemical behaviour of liquid mixtures3 and in the study of molecular interactions. Ultrasonic velocity of a liquid is related to the binary forms between the atoms or the molecules. Ultrasonic velocity have been adequately explained in understanding the nature of molecular interaction in pure liquids4 binary and ternary mixtures.5 The method of studying the molecular interaction from the knowledge of thermodynamic parameters and their excess values with composition gives an insight into the molecular process.6,7 The investigations regarding the molecular interaction in organic ternary liquid mixtures having aromatic hydrocarbon group as one of the compounds is of particular interest, since aromatic hydrocarbon group is highly polar and can associate with any other group having some degree of polar atoms. Ionic species play significant role in governing the physical, chemical and biological behavior of biological macromolecules like proteins, nucleic acids etc. Knowledge of thermodynamic mixing properties of binary mixtures has great relevance in theoretical and applied area of research. These data are needed for design processes in chemical, petrochemical and pharmaceutical industries. To explain many of the physico-chemical behaviour of a mixture of liquids, statistical thermodynamics has been widely used.8,9 Theories like corresponding theory, Flory’s theory, free length theory and collision factor theory have made significant contributions in the understanding of interactions in the liquid state.10,11 All these theories have been largely employed to explore the behavior of liquid mixtures. Since acoustic velocity is a thermodynamic function, many thermodynamic properties, especially the excess functions, can be deduced from a knowledge of this parameter. It is in this context that, the ultrasonic velocity measurement has become a key tool in the investigation of the behavior of liquid mixtures. From the measure value of sound velocity, isentropic compressibility can be found. Isentropic compressibility along with the thermal coefficient of expansion and the heat capacity at constant pressure, can be used to compute isothermal compressibility.12-20 The later may be employed to test the reliability of theories of liquids and liquid mixtures. Mahajan et al.21 reported the volumetric, viscometric and ultrasonic studies of some amino acids. Thermo acoustic studies of some electrolytic solvent mixtures are also reported.22 In the present study we are reporting the ultrasonic studies on binary and ternary mixtures of some organic liquids.
Experimental
An ultrasonic interferometer is used for the measurement of velocity. The measuring cell is filled with the experimental liquid and the high frequency generator is switched on. The ultrasonic waves moving away from the crystal are reflected back from the movable plate and stationary waves are formed in the liquid. The micrometer is turned slowly so that anode current on the micro ammeter falls to a minimum. The distance moved by the micrometer for the next minimum gives half the wavelength of ultrasonic waves. The total distance moved for n consecutive minima d is noted and velocity is calculated as v= λf, where λ is found from the relation d = nλ/2. Making use of Newton-Laplace equation
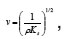
Results and Discussion
The thermodynamic excess functions of a composition of chloroform and ketones were studied in this work. The ketones used as acetophenone, cyclophexanone and ethyl methyl ketone. Ultrasonic velocity for the liquid mixtures are measured over a wide range of concentrations, using a single crystal ultrasonic interferometer at a frequency of 2 MHz. The binary and ternary mixtures studied are the following: (a) chloroform + acetophenone (b) chloroform + cyclohexanone (c) chloroform+ethyl methyl ketone (d) chloroform+ethyl methyl ketone+acetophenone and (e) chloroform + ethyl methyl ketone + cyclohexanone. Using Newton-Laplace equation, the adiabatic compressibility is calculated in each case. Values of thermal expansion coefficient and molar heat capacity at constant pressure are taken from the literature.23 For a mixture, the molar heat capacity at constant pressure and thermal expansion coefficient are assumed to be additive in terms of volume fractions and mole fractions. The various thermodynamic excess functions are given in tables 1 to 6.
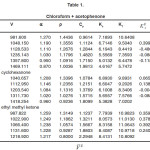
Table 1: Chloroform + acetophenone
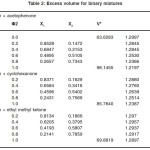
Table 2: Excess volume for binary mixtures
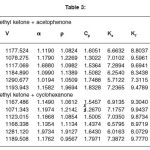
Table 3
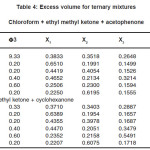
Table 4: Excess volume for ternary mixtures Chloroform + ethyl methyl ketone + acetophenone
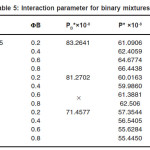
Table 5: Interaction parameter for binary mixtures
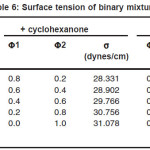
Table 6: Surface tension of binary mixtures
For the binary mixtures of chloroform + acetophenone and chloroform + cyclohexanone excess isentropic compressibility is negative for most of the concentrations. This means that the mixing is accompanied by a decrease in the intermolecular free space between the molecules. This can be explained in terms of the possibility of hydrogen bonding between the –CO- group and the hydrogen of the chloroform. For chloroform + methyl ethyl ketone, Ks is found to be positive for the whole range of composition. The positive deviation indicates that mixing is accompanied by an increase in the intermolecular free space. This may be due to a possible structure breaking effect. For all systems studied, the maxima in V E occur at XCHCl>0.5. The reason for this can be the following (i) in addition to AB hydrogen bonded complexes (A=chloroform, B=ketone) A2B complexes are also formed. This is theoretically feasible because of the presence of two lone pairs of electrons in the oxygen of the –CO- group. The existence of such complex formation can also be verified by measuring excess functions. (ii) The Van der Waal’s interaction between AB and B are stronger than the corresponding interaction between AB and A. The high value of for the mixture chloroform + cyclohexanone may be mainly due to the fact that the carbon atom of the –CO- group in cyclohexonone forms part of a six membered ring. Surface tension of the liquid mixtures also can be studied using Flory’s theory. Values obtained are of the same order of magnitude as those of similar types of liquids. For binary mixtures of chloroform with acetophenone and cyclohexanone, surface tension increases with increase in concentration of the ketone. But for the binary mixtures of chloroform and ethyl methyl ketone surface tension decreases with increase in concentration of the ketone. Therefore, from the experimental values of surface tension, the ultrasonic velocities and the composition dependence of the interaction parameter can be predicted, using this method of analysis.
References
- Oswel, S.L., Oswel, P., and Phalak, R.K., J. Sol. Chem. 27: 507 (1998).
- Rajasekar, J., and Naidu, P.R., J. Chem.Engg. Data, 41: 373 (1996).
- Sajnam, M.K., Indian J. Pure & Appl. Phys.38: 760 (2000).
- Kasare, S.B., and Patdai, B.A., Indian J. Pure & Appl. Phys. 25: 180 (1987).
- Ramasamy, K., and Ranganathan, V., Indian J. Pure & Appl. Phys. 27: 579 (1983).
- Jain, D.V.S., and Dher, N.S., Indian J. Tech. 310: 620 (1992).
- Langues, J.A.D., Hand Book of Chemistry, ed. 12, McGraw Hill, New York (1979).
- Prigogine, A., The Molecular Theory of solutions, North Holland Publishing Co., Amsterdam, (1957).
- Henderson, E., and Eyring, S., Statistical Mechanics and Dynamics, John Wiley and Sons, New York (1965).
- Flory, P.J., J. Am. Chem. Soc. 86: 3507 (1964).
- Flory, P.J., J. Am. Chem. Soc. 87: 1833 (1965).
- Seetharaman, T.S., Thermodynamic and Spectroscopic studies of Organic mixtures, Ph.D Thesis, University of Calicut (1978).
- Krishnaiah, A., Indian J. Pure & Appl. Phys. 19: 590 (1981).
- Srivastava, T.N., Indian J. Pure & Appl. Phys.21: 67 (1983).
- Ramamurthy, M., Indian J. Pure & Appl. Phys.21: 579 (1983).
- Naidu, G.R., and Naidu, P.R., Indian J. Pure & Appl. Phys. 22: 207 (1984).
- Pandey, J.D., Indian J. Pure & Appl. Phys. 25: 467 (1987).
- Gupta, M.C., Statistical Mechanics, Wiley Eastern, New Delhi (1990).
- Kumar, S., and Singh, V. B., Indian J. Pure & Appl. Phys. 30: 69 (1992).
- Kannappan, A.N., and Rajendran, V., Indian J. Pure. & Appl. Phys. 30: 240 (1992).
- Mahajan, A.R., Mirgane, S.R., Pagare, V.K., and Deshmukh, S.B., Met. Sci. Res. India 4: 345 (2007).
- Nithiyanantham, S., Mulkinathan, S., and Jayalakshmi, R., Oriental J. Chem. 25: 759 (2009).
- Tables of Physical and Chemical Constants, Longmans (1975).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal