Development and Validation of Novel Spectrophotometric Methods for the Determination of Ethacridine Lactate in Bulk
E. Venumadhav1, P. Sai Praveen2, T. Neha2, P. Bhargavi2 and G. Devela Rao2*
1Cheif Operating Officer, Veeda CR, Ahmedabad, Gujarat, India.
2KVSR Siddhartha College of Pharmaceutical Sciences, Vijayawada - 520 010, India.
Corresponding Author E-mail: drgdr1964@gmail.com
DOI : http://dx.doi.org/10.13005/msri/070220
Article Publishing History
Article Received on : 27 Jun 2010
Article Accepted on : 19 Jul 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Three simple and sensitive visible spectrophotometric methods (A-C) for the determination of Ethacridine Lactate in pure samples and pharmaceutical formulations are described. They are based on the formation of colored species by treating with Folin-Ciocalteau reagent (Method A, λmax 600nm) or 3-methyl 2-benzathiazolinone hydrazone in the presence of Ferric chloride (Method B, λmax 580 nm), or 1, 10 Phenathroline in presence of Ferric chloride (Method C, λmax 490nm). These methods were extended to the analysis of pharmaceutical preparations and results are compound with the refernce method (USP).
KEYWORDS:
Spectrophotometry; Ethacridine; Pharmaceutical preparation
Copy the following to cite this article:
Venumadhav E, Praveen P. S, Neha T, Bhargavi P, Rao G. D. Development and Validation of Novel Spectrophotometric Methods for the Determination of Ethacridine Lactate in Bulk. Mat.Sci.Res.India;7(2)
|
Copy the following to cite this URL:
Venumadhav E, Praveen P. S, Neha T, Bhargavi P, Rao G. D. Development and Validation of Novel Spectrophotometric Methods for the Determination of Ethacridine Lactate in Bulk. Mat.Sci.Res.India;7(2). Available from: http://www.materialsciencejournal.org/?p=2427
|
Introduction
Ethacridine lactate1,2 (EAL) is chemically 2-ethoxy-6, 9-diamino acridine monolactate monohydrate. It is used as an antispetic and also as an agent for second trimester tabortion.³ A survey of literature revealed only a few reported methods which include titrimetric,4 selective FTIR,5 visible spectrophotometry6 and HPLC methods for estimation of EAL in urine.7 The analytically important groups of EAL were not exploited for designing suitable spectrophotometric methods. Hence an attempt has been to develop simple and sensitive visible spectrophotometric methods for EAL determination for routine quality control analysis of EAL in formulations.
The developed methods are based on the formation of colored species by treating with Folin-Ciocalteau reagent (Redox reaction),8 3-methyl 2-benzathiazolinone hydrazone in the presence of Ferric chloride (Oxidative coupling)9 or 1, 10 Phenanthroline in presence of Ferric chloride (Oxidation followed by complexation).10
Experimental
Instrument
A systronics model 220 UV-Vis spectrophotometer with 1cm matched quartz cells were used for absorbance measurements
Reagents
All the chemicals used were of Analytical grade and prepared with double distilled water Aqueous solutions of FC reagent (2N) and 20% w/v Sodium carbonate solutions were used for Method A
Aqueous solutions of MBTH (0.2% w/v) and 0.01 % w/v Ferric chloride solutions were used for Method B.
1, 10 Phenanthroline Solution (0.198% w/v)
Prepared by dissolving 198 mg of 1, 10 Phenathroline in 0.1 N HCl and made upo the mark with 0.1 N HCl.
Ferric chloride solutions (0.0033M)
About 162 mg of anhydrous Ferric chloride was accurately weighed and dissolved in 100 ml of distilled water. 33.3 ml of above stock solution was further diluted to 100 ml with water.
Standard Drug Solution
Working standard solution of EAL was prepared by dissolving 10ml of the drug solution (1mg/ml) in water and made upto the mark of 100 ml with water (100 µg/ml).
Method A
Aliquots of standard EAL solution (100 µg/ ml) ranging from 0.5 to 2.5 ml were transferred to a series of 10 ml volumetric flasks. To each flask 3 ml of Sodium carbonate solutions and 1ml of FC reagent were added and kept aside for 5 min. The volume was made upto 10 ml with 0.1 N HCl. The absorbance was measured at 600 nm against reagent blank. The amount of EAL was deduced from its Beer-Lamberts plot.
Method B
Aliquots of standard EAL solution (100 µg/ml) ranging from 0.15 to 0.9 ml were transferred to a series of 10 ml volumetric flasks. To each flask 2 ml of Feeric chloride solutions and 1ml of MBTH reagent were and allowed to stand at room temperature for 15 min. The volume was made upto 10 ml with 0.1 N HCl. The absorbance was measured at 580 nm against reagent blank. The amount of EAL was deduced from its Beer-Lamberts plot.
Method C
Aliquots of standard EAL solution (100 µg/ml) ranging from 0.2 to 1.0 ml were transferred to a series of 10 ml volumetric flasks. To each flask 1 ml of Feeric chloride solutions and 1ml of 1, 10 Phenanthroline reagent were and the volumes in all the flasks were equalized with ethanol. The contents were gently boiled for 35 minutes, cooled to room temperature and 2ml of o-Phosphoric acid was added to all the flasks. The volume was made upto 10 ml with ethanol. The absorbance was measured at 490 nm against reagent blank. The amount of EAL was deduced from its Beer-Lamberts plot.
For Pharmaceutical Formulations
For injection formulations, 10 vials were broken and the constent were transferred to a clean and dried beaker. From this a volume of the drug solution equivalent to 100 mg of EAL was taken and a standard stock solution of 1mg/ml was prepared. This was appropriately diluted and the amount of EAL was found out as described in the procedure.
Recovery Study
To study the accuracy, reproducibility and precision of the proposed methods, recovery experiments were carried out. The recovery of the added standard was studied at 3 different levels. Each level was repeated 6 times. A plot of amount of drug found by proposed method (Y-axis) against standard added (X-axis) was drawn. The inrercept on Y-axis indicates the amount of drug present per formulation.
Results and Discussion
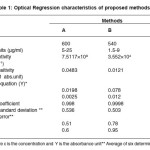
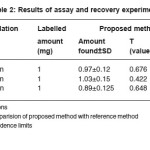
The optimum conditions for each method were established by varying one parameter at a time keeping the other fixed and observing the effect of product on the absorbance of colored species and incorporated in the procedure. The optical characteristics are given in Table 1, together with regression equation for calibration plots. The precision and accuracy were found by analyzing six replicate samples containing known amount of drugs and the results were summarized in Table 1 & 2. shows that the values of % recovery are between 98 to 102% and the value of coefficient of variation are sufficiently low indicating that the proposed methods are free of interferences from any excipients like strach, talc etc and the results are reproducible. Thus these developed methods can be employed for the routine determination of EAL in pure and in pharmaceutical preprations.
Table 1: Optical Regression characteristics of proposed methods
Table 2: Results of assay and recovery experiments
References
- United States Pharmacopoeia 24, national formulary 19, section 1225 “Validation of compendial methods, US Pharmacopoeial convention, Rockville (2000).
- European Pharmacopoeia, 3rd edition, 1997 and Supplement, Council of Europe Strasburg (1999).
- A. Malpani and U. Krishna, “Second trimester abortion with ethacridine lactate plus Carboprost”, Advance in contraception., 4(2): 154-157 (1987).
- T. Sakai, N. Miyatake and N. Ohno, Appl. Spectrosc., 50: 1203 (1996).
- Bratu, I. Chiosa V, Stanculescu, Annal of West University of Timisoara, Series of Chemistry, An International Journal. 46: 951(1997).
- R.K. Gilpin, L.A., Pachla, Anal. Chem. 67(12): 295-313 (1995).
- Zhi-Yong Guo, Dan-Yi Wei, Yuan-Yuan Wang, Kun-Fei Xuan, Xu-Fei Yu, Qui-Luan Yu, Yun Chu “An HPLC method for the determination of ethacridine lactate in human urine “Biomedical chromatography, 21(5): 480-483 (2007).
- CSP Sastry and BS Sastry “Application of MBTH chemistry in the determination of pharmaceuticals”, The Easter Pharmacist 30-33 (1986).
- K.P.R. Chowdary and G. Devala Rao, “Spectrophotometric determination of Salmeterol xinafoate with Folin-Ciocalteau Reagent, “Indian Drug”. 34(10): 606-607 (1997).
- S. Vijaya Saradhi, V. Hima Bindu and G. Devala Rao, “New Spectrophotometric methods for the determination of Simvastatin”. Acta Ciencia Indica, XXXIIIC, No 2: 205 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal