Rahul Bhola*, Shaily M. Bhola, Brajendra Mishra and David L. Olson
Department of Metallurgical & Materials Engineering, Colorado School of Mines, Golden, Colorado - 804 01, USA.
Corresponding Author E-mail: bhola.rahul@gmail.com
DOI : http://dx.doi.org/10.13005/msri/070210
Article Publishing History
Article Received on : 08 Oct 2010
Article Accepted on : 17 Nov 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
The microbiologically influenced corrosion (MIC) is one of the most common forms of corrosion that results from the presence and activity of microorganisms. The presence of microorganism aids in the formation of a bio film and constitutes various bacterial cells, extracellular polymeric substrates (EPS) and corrosion products. In this paper, a review on the importance of MIC and various ways to mitigate has been introduced; a brief description of the physical, chemical, electrochemical and biological mitigation methods for MIC has been included and EPS formation mechanism, chemical composition, properties and its influence on corrosion has been discussed.
KEYWORDS:
Microorganisms; MIC; EPS; Corrosion
Copy the following to cite this article:
Bhola R, Bhola S. M, Mishra B, Olson D. L. Microbiologically Influenced Corrosion and its Mitigation: (A Review). Mat.Sci.Res.India;7(2)
|
Copy the following to cite this URL:
Bhola R, Bhola S. M, Mishra B, Olson D. L. Microbiologically Influenced Corrosion and its Mitigation: (A Review). Mat.Sci.Res.India;7(2). Available from: http://www.materialsciencejournal.org/?p=2388
|
Introduction
Microbiologically Influenced Corrosion (MIC)
Microbiologically induced corrosion (MIC) has been reported in several papers and journals in the literature. It is quite clear that the microorganisms affect the corrosion of metals and alloys immersed in aqueous environments.1 Microbes adhere to the metal surfaces forming biofilms, which can alter the surface chemistry at the interface. Biofilms or the microbial coverings form complex ecosystems in the presence of the four prerequisite of life, i.e., a small amount of water; an electron donor; an electron acceptor and a carbon source.2 A single bacterial species can form a biofilm, but more often biofilms consist of many species of bacteria and may include fungi, algae, protozoa, cellular exudates/ extracellular substrate (EBS), corrosion debris and by-products.3
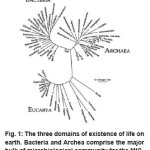
The existence of life on earth has been categorized into three main doamins, i.e., the Bacteria, the Archaea and the Eucarya, which have been shown in a schematic in Fig. 1. The Eucarya comprise of the plants, animals and fungi. However, the bulk of life on the planet is still composed of microbial organisms from the Bacteria and Archaea domains. Even the majority of Eucarya are microbial- for instance, Giardia a well-known protozoan microbial Eukaryote.3
Figure 1: The three domains of existence of life on earth. Bacteria and Archea comprise the major bulk of microbiological community for the MIC
In the oil and gas industry, several types of bacteria’s are found that cause increased damage to pipelines and tanks transmitting loads of fuel. The sulfate reducing bacteria (SRB) takes the major lead. These bacteria, reduces sulfate to sulfide, are anaerobic and can thrive in the temperature ranging from 25 to 60 °C. Several other types of SRB’s can reduce nitrates, sulfites, thiosulfates and fumarates with organic compounds or H2.4 Microbial flora can also oxidize manganese, iron and various other elements present in the pipes to reaction products, such as MnO2, FeO, Fe2O3, MnCl2, FeCl2 and favor corrosion. A complex mechanism of interaction occurs between the aerobic and anaerobic bacteria further promoting corrosion. Due to the deposition of biofilm or plaque deposits, the metal surface beneath is exposed to different amounts of oxygen causing the creation of differential aeration cells. Less aerated zones act as anode, which undergoes corrosion releasing metal ions. These metal ions combine with end product of bacteria, along with the chloride ion of electrolyte to form more corrosive metal halides like the MnCl2, FeCl2 and so on further aggravating the attack.5 Microbial corrosion is intensified when the acidic metabolic products of microbes and bacteria further corrode metal surfaces.
It is estimated that microbiologically influenced corrosion is an issue worth 30 billion dollars annually, contributing to 20 % of all the corrosion problems faced by North America’s oil and gas industry5. MIC have also been found in aqueous environments, oil wells and transmission lines,6 spreading all over the North America and the globe.
Extracellular Polymeric Substrates (EPS)
Majority of the microbial flora live and grow in biofilms, where they secrete a polymeric gel like matrix and is known as the extracellular polymeric substrate (EPS). EPS is the main component that establishes the structural and functional integrity of the biofilm and plays a substantial role in determining the physiochemical and biological properties of biofilm. EPS can be as high as 90% of the biofim7-13. It is defined as a biosynthetic polymer. Wingender et al. termed EPS as the organic polymers of microbial origin which in biofilm system are frequently responsible for binding cells and other particulate materials together (cohesion) and to the substratum (adhesion)8. The contribution of EPS in the growth of biofilm was first pointed by Neu and Marshall8 in 1990. It can also be defined as the bound EPS (like sheaths, condensed gels, loosely bound polymers, capsular polymers and attached organic material) and the soluble EPS (such as soluble macromolecules, colloids and slime).8
Composition of EPS
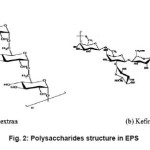
Depending upon the environmental conditions and microbial flora present, EPS can vary significantly from a homogenous to a more heterogeneous composition of the extracellular polymers. Proteins (glycoproteins), carbohydrates and humic acid substances are the main organic fractions of the EPS10. 40-95 % of the components of the macromolecules in the microbial EPS are accounted for the exopolysaccharides.11 The high molecular weight homopolysacchride, dextran is produced by the gram-positive bacteria Firmicutes. Firmicutes produces another type of polysaccharides, known as kefiran. The structures of dextran and kefiran have been shown in Fig. 2.14
Figure 2: Polysaccharides structure in EPS
EPS Formation Mechanism
The gene regulation, quorum sensing (QS), is the release of a chemical signal from a bacterium, in response to a change in the cell density. This chemical signal molecule is known as autoinducer. The autoinducers concentration changes as a function of the cell density15. QS plays a significant role in regulating the mechanism of the EPS production, colonization, biofilm formation and differentiation. When a bacterium approaches a surface it senses the surrounding environment and releases protons or other signaling molecules into the bulk solution. These protons and signaling molecules are known to diffuse away from the floating cells. As the floating cells density increases, the concentration of protons and signaling molecules also increases as a result of diffusion limiting the process. Living cells are attracted to surface and they can sense these surfaces by the change in concentration of protons or signaling molecules. When the planktonic cells sense the surface, they start to adhere to the surface and form biofilm. In order for these cells to maintain the adhesion process, they must biosynthesize certain adhesive polmers in the form of some exopolysaccharides. These materials help the cells to adhere to the surface and to other bacterial cells and enhance the irreversible adhesion phase of biofilm formation.16
Formation of Biofilm
The biofilm develops in three stages, attachment, colonization and detachment. When microorganisms interact to a surface, they immediately produce slimy adhesive substances (EPS), which enhance the formation of microcolonies and biofilm. EPS helps the cells to adhere to the surface and to the other cells. EPS can also control the substratum-biofilm interfacial chemistry and can regulate the cohesive forces within the biofilm and biofilm stability.
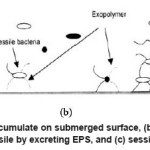
In process industry, chlorination is a common method of controlling biofilm formation. Transport of chlorine to the biofilm is a diffusion controlled process, which depends on the concentration of chlorine in the bulk solution and the amount of turbulence. Microbial cells are inactivated by the chlorine in water, which also oxidize the nutrients. Chlorine also reacts with organic and inorganic compounds in the biofilm, which results in the disruption of cellular material and inactivation of cells. Figure 3 shows the influence of EPS on the attachment of planktonic bacteria to the surface.17-21
Figure 3: (a) conditioning film accumulate on submerged surface, (b) planktonic bacteria colonize on the surface and become sessile by excreting EPS, and (c) sessile cells replicate on the surface
Properties of EPS in a Biofilm
The large amount of water incorporated inside the extra cellular polymeric substrate by the hydrogen bonding makes it highly hydrated. EPS can be hydrophobic or hydrophilic and have different solubilities in water depending upon the surface polarities of the polysaccharides. The conformation of EPS depends on the composition and the structure of the polysaccharides. The presence of the 1,3 or 1,4-b-linked hexose residues in the EPS backbone structure makes it rigid, un-deformable and insoluble.17-18 The availability of nutrients in the growth media has a substantial effect on the production of EPS, for example, the excess of carbon and depletion of nitrogen, potassium, can enhance EPS formation. EPS production is also encouraged by slow growth microorganisms. EPS prevent dehydration of biofilm by its hydration property. It can resist the antibiotics by impeding their mass transport through the biofilm or by binding to those antibiotics.19-22
Microbiologically Influenced Corrosion Mitigation
MIC can be controlled in several ways, 1) physical methods, 2) chemical methods, 3) electrochemical methods and 4) biological treatments. The following section summarizes the uses, advantages and disadvantages of these treatments.
Physical Methods
The extenuation of MIC, using physical treatments, involves the use of mechanical forces to scrap of the biofilm and is not only limited to pigging, but also involves the use of ultraviolet radiations and ultrasonics.
Pigging
A pig is a tool that is used inside the oil and gas containing pipes for cleaning. Most of the time pigs are used to inspect, clean, and add chemicals to the pipeline. Pigs work well in cleaning and inspection of pipeline.21 If a biofilm is formed inside the pipeline, pigging can scrape it out, but it cannot remove the entire bacterial colonization and the bacteria may grow and form the biofilm again. During localized corrosion in addition to MIC, bacterial cells penetrate inside the pits and the brush of the pigs is not able to penetrate the pits inside the pipes and clean effectively. Although pigging is the recommended nondestructive technique for inspection and cleaning of the pipelines, it is still not the ideal method to prevent MIC.
Ultraviolet Radiation
When treating with ultraviolet radiations, the DNA of the microorganisms is altered in a way that they cannot produce byproducts which are responsible to cause corrosion22. Although UV shows a high killing efficiency, but it only works for the surfaces exposed directly to the radiation and it is difficult to manage such exposure in the long pipelines. UV can be blocked by some microorganisms, resulting in a less efficient or higher dose of UV light, required for a higher killing rate. It has been reported that some microorganisms are not affected by the UV radiations.21
Ultrasonic Treatment
Ultrasonics has been recognized since several decades to destroy cell wall and cell membrane of the microorganisms. The mechanism for the ultrasonics is that it applies an acoustic pressure (Pa) and subsequent cavitation in the treated bubbles in the liquid. These cavitation bubbles, when collapse, increase the pressure in the order of hundreds of atmospheres and the temperature in the order of thousands of degrees. The treatment shows, a promising killing efficiency greater than 99 %. Cavitation, can also cause damage to the substrate surface and may aggravate crevice corrosion.23
Chemical Methods
Biocides are the most effective chemicals used to terminate microbiologically influenced corrosion. They are chemical compounds, which kill living microorganisms, and are of two types – oxidizing biocides and non-oxidizing biocides.
Oxidizing biocides, like chlorine, can penetrate and destroy the cell, but the non-oxidizing biocides such as aldehydes can also penetrate and destroy the cell membrane. Biocides that can kill many diverse types of microorganisms are known as broad-spectrum biocides21. Biocides can sometimes cause a subsequent corrosion problem on the structural materials. They can be expensive and unfriendly to the environment.
Electrochemical Methods
Applying negative potential (cathodic protection) to the structural materials is one of the most common methods for corrosion protection. It can reduce/prevent the corrosion of any metal/alloy electrolyte combination in a given industrial application. Several mechanisms explaining the corrosion prevention on cathodic polarization has been summarized in literature, and the most common theory supporting microbial corrosion prevention has been described below.24
Electrostatic-Chemical Theory
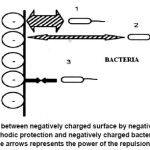
On applying a negative potential on a metal during cathodic protection, the surface becomes more negatively charged and repels the negative charged bacteria as shown in Figure 4. These repulsions prevent the bacteria from attaching to the surface until the distance is in a nano range. At the same time, the cathodic reaction happening over the metal, effects the hydroxyl ion release, thereby increasing the pH, which will subsequently make the environment more alkaline and slow down bacterial proliferation.21
Figure 4: Repulsion between negatively charged surface by negative polarization of a metal during cathodic protection and negatively charged bacteria. The thickness of the arrows represents the power of the repulsion force
Coating
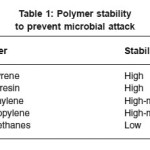
Almost all of the underground pipeline are coated with some form of coating. Coating disbandment is usually the commonest cause that leads to corrosion failure in this field. For a coating to be effective, it should be adherent, coherent, non-porous and mechanically resistant. Some of the coatings are polymer material. Table 1 summarizes the stability of some polymers to the microbial attack.21 It is also essential to have a continuous protective coating that can tolerate aggressive chemicals such as sulfuric acid and acetic acid, which are produced by some acid producing bacterial as a part of corrosion attack.
Table 1: Polymer stability to prevent microbial attack
Biological Treatments
The most recent scheme for the mitigation of microbiologically, influenced corrosion using biological treatments, is by the use of different types of bacteria against the primary bacteria responsible for MIC in a specific industrial environment. Some strains of SRB are capable of reducing nitrate and some authors propose using nitrate reducing bacteria to exclude SRB. Nitrate can inhibit SRB or affect the enzyme that SRB used to reduce sulfate to sulfide.4
Conclusion
Microbiologically influenced corrosion is a serious corrosion problem in different fields.
Extracellular polymeric substrate has a substantial role in the adhesion of microorganisms to the surfaces and to the other cell.
The chemical composition and the conformation of exo-polymers in EPS may show a discrepancy from one type of bacteria to another and also the environmental conditions under exposure.
MIC can be treated by different techniques. However none of them has a100 % efficiency.
References
- Peng CG, Park JK, Principal Factors Affecting Microbiologically Influenced Corrosion of Carbon Steel, Corrosion, 669-675 (1994).
- Spear J, Walker J, Pace N, Microbial Ecology and Energetics in Yellowstone Hotsprings, Yellowstone. Science, 17–24 (2006).
- Almahamedh H, Williamson C, Spear J, Mishra B, Olson DL. Characterization of Microbiologically Influenced Corrosion on Carbon Pipeline Steels Using Advanced Analytical Techniques. Paper No.88, Leeds, UK (2008).
- Zhu X, Modi H, Kilbane J, Efficacy and Risks of Nitrate Applycation for the Mitigation of SRB-Induced Corrosion, NACExpo, paper No. 06524 NACE, Houston, TX, 1-41, (2006).
- Setareh M, Javaherdashti R, Precision comparison of some SRB detection methods in industrial systems, Material Performance, 60-63 (2000).
- Tatnall R, Fundamentals of Bacteria Induce Corrosion, Materials Performance, 32-37 (1981).
- Christensen BE, Characklis WD. Physical and Chemical properties of biofilms. In: Characklis and Marshall (eds) Biofilms. Wiley, New York. 93-130 (1990).
- Wingender J, Neu TR, Flemming HC. Microbial Extracellular Polymeric Substances, Characterization, Structure and Function. Springer, New York (1999).
- Wagner M, Ivleva NP, Haisch C, Niessner R, Horn H. Combined use of confocal laser scanning microscopy (CLSM) and raman microscopy (RM): investigation on EPS-Matrix. Water Research,43: 63-76 (2009).
- Morgan JW, Evison LM, Forster CF. An examination into the composition of extracellular polymers extracted from anaerobic sludge. Trans Chem partB, 69: 231-236 (1991).
- Pal, Paul AK. Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J. of Microbiology, 48:49-64 (2008).
- Donlan RM, Biofilms: Microbial life on surfaces. Emerging Infect. Di, 8: 881-890 (2002).
- Flemming HC, Wingender J. Relevance of microbial extracellular polymeric substances (EPSs)–Part I: Structural and ecological aspects. Water Sci. Technol, 43: 1-8 (2001).
- Barbara VU, Chen M, Crawford JR, Ivanova EP. Bacterial Extracellular Polysaccharides Involved in Biofilm Formation. Molecules, 14: 2535-2554 (2009).
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annual review of microbiology, 55: 165-199 (2001).
- Costerton JW. Introduction to biofilm. International J. of Antimicrobial Agents, 11: 217-221 (1999).
- Beech B, Campbell SA, Walsh FC. The role of surface chemistry in SRB influenced corrosion of steel. Int. J. Marine Biol. Oceanog., OEBALIA, 13: 243-249 (1994).
- Hussain M, Wilcox MH, White PJ. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol Rev, 104: 191-208 (1993).
- Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J, 46: S47-S52 (2000).
- Little BJ, Lee JS. Microbiologically influenced corrosion. Wiley, New Jersey (2007).
- Javaherdashti R. Microbiologically Influenced Corrosion, an engineering insight. Springer. London (2008).
- Almajnouni D, Jaffer AE. Monitoring microbiological activity in a wastewater system using ultraviolet radiation as an alternative to chlorine gas. Corrosion, NACE international, paper No. 03067, (2003).
- Pound BG, Gorfu Y, Schattner P, Mortelmans KE. Ultrasonic Mitigation of Microbiologically Influenced Corrosion. Corrosion, 51(5): 452 -463 (2005).
- Stein A. MIC treatment and prevention. In: a practical manual on MIC Kobrin (ed) NACE Houston Texas USA (1993).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal