A Thermo Optoacoustic Analysis to Detect Photochemical Reaction of Medicine Ciprofloxacin Hydrochloride
K. Krishnankutty1, G. Krishnakumar2 and S. Mohanan1
1Department of Physics, University College,Thiruvananthapuram, Kerala, India.
2Department of Physics, College of Engineering, Thiruvananthapuram, Kerala, India.
DOI : http://dx.doi.org/10.13005/msri/080122
Article Publishing History
Article Received on : 10 Apr 2011
Article Accepted on : 26 May 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
A new thermo-optoacoustic analysis has been used to detect the photo chemical reactions in medicine ciprofloxacin, a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. Certain derived optoacoustic parameters of pure medicine and that exposed to solar radiations have been determined as a function of temperature. Plots of these parameters versus temperature have been made. The nature of variation and the relative shift in the curves for the exposed samples compared with the un exposed ones have been used to study photo chemical reactions. The results have been explained in the light of existing theories and confirmed using UV absorption spectra of the samples.
KEYWORDS:
Thermo-optoacoustic analysis; Non destructive method; Photochemical reaction; Ciprofloxacin
Copy the following to cite this article:
Krishnankutty K, Krishnakumar G, Mohanan S. A Thermo Optoacoustic Analysis to Detect Photochemical Reaction of Medicine Ciprofloxacin Hydrochloride. Mat.Sci.Res.India;8(1)
|
Copy the following to cite this URL:
Krishnankutty K, Krishnakumar G, Mohanan S. A Thermo Optoacoustic Analysis to Detect Photochemical Reaction of Medicine Ciprofloxacin Hydrochloride. Mat.Sci.Res.India;8(1). Available from: http://www.materialsciencejournal.org/?p=2563
|
Introduction
Sunlight is an important cause of damage to medicine and other organic materials. Fluorescent lamps also play the same role. Short wavelength ultra violet radiation has long been recognized as the reason for most of this damage. We know that the time required for the occurrence of a photo chemical reaction is very short. So the possibility of a photo chemical reaction of the medicine due to sunlight or fluorescent light during the consuming process cannot be neglected. In surgical operation theatre, powerful fluorescent lamps may be used to illuminate the operating part. In this way photo chemical reaction1,2 may take place on the medicine circulating through exposed blood vessels. Degradation or loss of activity can occur with exposure to light.3,4 It is known that improper storage conditions and patient ignorance about usage are responsible for exposure to radiations. So proper storage facilites and advice to patients ensure safety of light sensitive medicines. It is noted that un protected medicines exposed to fluorescent lamps or even sunlight for several weeks were administered to the patient. The most common consequences of drug photodecomposition is loss of potency with concomitant loss of therapeutic activity. Even if a drug product is shown to be photo-chemically inert, it can still acts as a source of free radicals or form photo toxic metabolites. So a detailed study of light sensitive medicines are essential in order to keep them secure during administration. An aspect of major importance in understanding the pharmco-kinetic properties of drugs and the mechanism of their action is that of acid-base equlibria. According to the definition of Lowry and Bronensted, acids are proton donors and bases are proton acceptors. On deprotonation5 the acid yields the conjugate base,whereas protonation converts the bases into its conjugate acid. An orally administered drug will most likely change its protonation state during its passage through the acidic stomach and the alkaline intestinal tract. Thus photochemically transformed medicine is administered to a patient, this change will influence its solubility in an aqueous enviornment, its permeability through membranes and last, but not least, its binding constant to the receptor site. Thus activity of the medicine gets reduced.
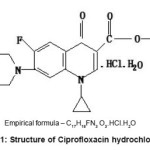
We are engaged in a systematic study of liquid state using some opto-acoustic parameters. In the present work,thermo opto-acoustic analysis,6-8 a simple and non destructive method compared with expensive and time consuming methods such as chromatographic and enzymatic techniques,9 is done to study the photo chemical reactions of a medicine ciprofloxacin and the same was confirmed using UV spectroscopy. In the present work, we have used ciprofloxacin hydrochloride, eye drops known by the trade name ciplox, structure of which is given in fig 1. Ciprofloxacin hydrochloride, a fluoroquinolone, is the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7- (1-piperazinyl)-3-quinolinecarboxylic acid. It is a second generation fluoroquinolone antibacterial. It kills bacteria by interfering with the enzymes that causes DNA to rewind after being copied, which stops DNA and protein synthesis.
In thermo opto-acoustic analysis, the opto-acoustic parameters of a liquid are plotted as a function of temperature. A pure liquid (single or multicomponent) has a characteristic curve depending on the chemical composition of it. Any deviations from this may be taken as an indication of change in chemical combinations due to reactions induced by external agencies. This is the basic principle used in thermo-opto-acoustic analysis. This method has been used successfully to distinguish between fructose and glucose (isomers having the same chemical composition), to detect and quantify the amount of fructose in coconut water,7 to detect the adulteration of milk,8 to study the effect of sunlight on medicine disodium hydrogen citrate10 and to detect photochemical reaction of medicine salbutamol.11 In the present work, we have studied the effect of solar radiations on the photochemical changes of the medicine ciprofloxacin
Experimental
Three samples of medicine ciprofloxacin were selected for the experiment. Sample A is the pure medicine manufactured by Cipla Ltd. Sample B was prepared by exposing the pure medicine to scattered sun light entering through the open window of the laboratory for 2hour from 12:30 pm to 2:30 pm on a sunny day. The optical power of scattered sunlight measured was 9.0 dB. Sample C was prepared simultaneously by exposing the pure medicine to the direct sunlight outside the laboratory on the same day for the same duration. The optical power of direct sunlight measured was 31.2 dB. The measurements were done using an optical power meter manufactured by INFOS model-100 The experiments were carried out immediately after preparing the samples. The density(r), Ultrasonic velocity (U) and refractive index (n) were determined at five different temperatures 298.15, 303. 15, 308.15, 313.15 and 318.15K. The temperature was kept constant using a thermostatically controlled water circulating arrangement with an accuracy of ± 0.1K. Density measurements were performed using a 12 cm3 double stem pyknometer. Masses were measured using a single pan electronic balance with an accuracy of ± 0.1 mg. Ultrasonic velocities were determined by a single crystal ultrasonic interferometer0.15 at a constant frequency of 2 MHz with an accuracy of ± 0.1m/s. The refractive index was measured using Abbe’s refractometer with an accuracy of ± .0001. All the precautions were taken to minimize the possible experimental error. The setup is checked for standard liquids and liquid mixtures. The values obtained are compared with earlier values and found that they match very well with each other.
Though direct parameters such us ultrasonic velocity(U), density(ρ) and refractive index(n) can be used for the analysis, studies on liquids and liquid mixtures reveal that, derived opto-acoustic parameters are more useful. The derived opto-acoustic parameters used in the present work are viz, specific opticimpedance(Z0),12 specific opto-acoustic velocity(η),6-8 specific opticalvolume6(υ), adiabatic compressibility (βs)13 and were calculated using equations



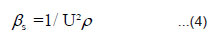
The UV absorption spectra of the samples A, B and C were taken using a double beam UV visible Spectrophotometer-2202 operated in the scan mode supplied by Systronics India. The derived Opto-acoustic parameters Zo, h , υ, and βs are plotted against temperature for pure medicine and the medicine exposed to sunlight.14 The nature of variation and the relative shift7,8 in the curves for exposed samples from the unexposed one have been used to detect the photo chemical change and hence the change in the activity of the drug. The results are explained using the UV absorption spectra of the pure and the exposed medicine.
Results and Discussion
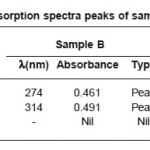
The UV absorption spectra peaks of samples A,B and C are given in table 1 and graphs plotted with Zo, h, υ, and βs versus temperature are shown in figs 5 to 8 respectively.
Table 1: UV absorption spectra peaks of samples A,B and C
The pure medicine (sample A) shows an absorption maximum at 274 and 316 nm. This corresponds to the π-π* transition and n-π* transitions of the carbonyl group conjugated with the double bond in the compound. The π-π* transitions of the benzene nucleus is also accounted by this absorption. On exposure to scattered light (Sample B) the absorption at 316 nm is shifted to a lower value 314 nm. Hence there is no change in the mode of transitions and the same π-π* and n-π* of the conjugated carbonyl group is accounted. But here the absorbance is modified that indicates a slight variation in the molecular population in the ground and excited states. But during ir-radiation by dim light there is no possibility of photochemical change in the lead molecule of the medicine.
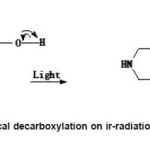
On exposure to bright light (Sample C), there is a shift of λmax 274 nm to a longer wavelength region λmax 290 nm. The other bands were not affected except with the appearance of a new band at λmax, 206 nm. So the π- π* transitions of the aromatic nucleus becomes more stronger in bright light. More over π-π* and n-π* transitions has taken place at longer wavelength 290 nm. The probability is that the lead molecule can undergo photochemical decarboxylation forming an α-β unsaturated cyclic ketonic system in which the energy gap between highest occupied molecular orbital and lowest un occupied molecular orbital decreases and the wavelength absorbed is increased to a higher region. Since there is an increase in the absorbance indicates that the molecular population in the excited state also increased. As the duration of exposure to bright light increases the decarboxylation tendency also increases. As the exposure of light continues, after eliminating COOH group, further strong interaction with bright light leads to photochemical decomposition of carboxyl group.ie photochemical decarbonylation takes place. Thus photochemical change of the structure of the lead molecule is possible in bright light and the activity of the medicine can change during ir-radiations by bright light.
Figure 1: Structure of Ciprofloxacin hydrochloride
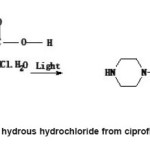
Figure 2: Elimination of hydrous hydrochloride from ciprofloxacin hydrochloride
Figure 3: Photochemical decarboxylation on ir-radiations by bright sunlight
Figure 4: Photochemical decarbonylation on ir-radiation by bright sunlight
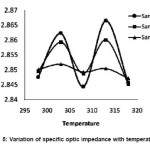
In figure 5, the specific optic impedance is plotted as a function of temperature for the samples A, B and C. For sample A, the graph is having nearly a sinusoidal variations with peaks at 303 and 313 K and dip at 308K. Snce Zo involves the ratio of ρ and n and ρ, a structure independent and n, a structure dependent parameter. So the moderate variation of Zo with temperature of the original sample indicates the structural changes due to changes in the molecular association between the molecules when thermal variation is applied to original sample. When the sample is exposed to scattered sunlight, the variation of Zo with temperature again follows a sinusoidal variations with peaks at 303K and 313K and dip at 308K but the curve is slightly shifted downward. The slight downward shift shows a small rearrangement of molecules within the medicine and thereby change the refractive index of the medicine due to ir-radiations by scattered sun light.
Figure 5: Variation of specific optic impedance with temperature
When the sample is exposed to direct sunlight (sample C), the curve is more shifted downward and gets smoothened when it is ir-radiated with bright light, the lead molecule can undergo photochemical decarboxylation forming an α-β unsaturated cyclic ketonic system and the new structure formed is temperature insensitive and therefore the sample C showed an inert thermal behavior. This inert thermal behavior, dissimilarity in the curve compared to samples A and B and large downward shift indicates clearly a photochemical transformation occurred to the original sample.
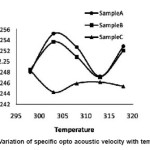
In fig 6, opto-acoustic velocity is plotted as a function of temp. In sample A, the graph is having positive slope till 303K and beyond 313K and shows a negative slope up to 313K. Here also, the curve has nearly a wave like variations. This oscillatory nature of the curve indicates variations in the ultrasonic velocity of the original sample due to variations in the intermolecular association between the molecules of the medicine. In sample B, the curve is slightly downward shifted and the variation is nearly as that of sample A. The slight downward shift is due to increase in the molecular population of the molecule and thereby ultrasonic velocity gets decreased. In sample C, the curve is very well downward shifted and shows a small negative slope till 303K and then shows a small positive slope till 308K and then shows nearly an inert thermal behavior. This large downward shift is due to large increase in the population of the molecules in the excited state and thereby causes an increase in the intermolecular free length and then produces a decrease in the ultrasonic velocity. This contributed to the decrease in optoacoustic velocity. The slight increase of ç with temperature in the region 303K to 308K is due to decrease of refractive index with increase of temperature. When the temperature is increased further ç stabilizes due to the principal effective absorption in the ultraviolet region. This absorption band component of refractive index increases as temperature increases and these two effects gets nullified and this contributed to the near inert thermal behavior in this region. This downward shift and variations in the nature of the curve compared to A and B indicates a photochemical transformation in the sample C. This is very well confirmed by UV spectra.
Figure 6: Variation of specific opto acoustic velocity with temperature
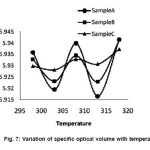
In fig 7 specific optical volume is plotted as a function of temperature. For sample A, the curve has nearly a wave like variations; this is the characteristic thermal response of í for the medicine ciprofloxacin. This characteristic thermal response may be due to change in the strength of the intermolecular association and related structural changes. When the sample is exposed to scattered sunlight, there is a small upward shift and the curve follows a wave like variations with little damping. This upward shift may be due to π-π* and n-π* transitions of the conjugated carbonyl group increases with scattered radiations. These small variations are due to the small chemical changes of the medicine due to the photochemical reaction in scattered sunlight. When the sample is exposed to direct sunlight (Sample C), the curve is more upward shifted and exhibits nearly an inert thermal behavior. This characteristics may be due to large increase of π-π* and n-π* transitions of the aromatic nucleus and conjugated carbonyl group and the lead molecule can undergo photochemical de-carboxylation and the new structure formed is nearly temperature insensitive. This upward shift and dissimilarity in the curve for sample C from that of sample A and B clearly indicates photochemical transformations in the original sample when it is exposed to direct sunlight.
Figure 7: Variation of specific optical volume with temperature
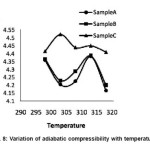
In fig 8, adiabatic compressibility is plotted as a function of temperature. In sample A, the curve has a negative slope till 303K and beyond 313K and has a positive slope between 308k and 313K. The adiabatic compressibility is a measure of intermolecular association between the molecules of the medicine15 and the study of the change in the molecular association between the molecules reveals interaction of chemical groups with biological receptor sites. When the sample is exposed to scattered radiations, the curve is slightly upward shifted and has similar variations as that of sample A. This small upward shift is due to change in the behavior of the electronic excited state formed when photon is absorbed by functional group. As a result such excitation results in a change in molecular orbital occupancy, an increase in energy and change s in local bonding and charge distribution. When the sample is exposed to direct sunlight, the curve has a large upward shift till 303K, then has a negative slope and then stabilizes it nearly. Here the maximum absorption is moving to longer wavelength as the amount of delocalization increases and there must be less energy gap between the bonding and antibonding orbital’s as the amount of delocalization increases. There must be large population of molecules in the excited states, causing decrease of ultrasonic velocity and there by increased adiabatic compressibility. The dissimilarity in the curve, upward shift and near inert thermal behavior clearly indicates photochemical transformation of the medicine.
Figure 8: Variation of adiabatic compressibility with temperature
Thus the original compound is sensitive to sunlight and other fluorescent UV light and there is a possibility of photo chemical decomposition. So continuous exposure to UV lights probably decrease the actual concentration of the original compound due to this photo chemical transformation and the biological activity of the medicine gets suppressed during irradiations. There for patients should take extra care while consuming the medicine in the presence of fluorescent lamps or sunlight. Since the biological activity of the drug molecules and the activation energy of the metabolic process basically depend upon the type and strength of the intermolecular interactions.16 There for thermal variations of Zo, η, υ, and βs for the pure medicine provides valuable information regarding action of medicine on human body. Moreover the nature of variation and the relative shift in the curves for exposed samples from the unexposed have been used to detect the photo chemical change and hence change in the activity of the drug. There for opto-acoustic analysis is a powerful tool in understanding the activity of the pure medicine and the photo chemical changes in the exposed samples in a non –destructive way using ordinary non-sophisticated instruments.
Conclusion
Thermo opto-acoustic studies on pure medicine Ciprofloxacin reveals physico-chemical behavior of the medicine by studying its characteristic curves. But dissimilarity and relative shift in the curve for exposed samples from unexposed one reveals photo chemical transformation in the exposed samples. So human exposure to sunlight after consuming the medicine may result in a significant reduction in both cutaneous and circulating level of medicine ciprofloxacin.
References
- Cosa, G., J. Pure. Appl. Chem., 76(2): 263 (2004)
CrossRef
- Rohtagi, K., and Mukherjee, K., Fundamentals of Photochemistry,Wiley Eastern Ltd., Delhi (1986)
- Yoshioka, S., and Stella, V.J., Stability of drugs and dosage forms, Springer
- Miranda, M.A., Pure Appl. Chem., 173(3):481 (2001).
CrossRef
- Odian, G., and Blei, I., General, Organic and Biological Chemistry,Tata Mcgraw –Hill.
- Mohanan, S., Vaidyan, V.K., and Kurian, K.V., Acustica Acta Acustica, 83: 367 (1997).
- Mohanan, S., Iype, L., Laila, M., Panicker, P.G.T., Bindu, R.G., and Domini, I., J. Acoustic Lett., 24(9): 171 (2001).
- Mohanan, S., Panicker, P.G.T., Iype, L.,Domini, I., Laila, M., and Bindu, R.G.,Pramama J.Physics, 59(3): 525 (2002).
- Egan, H., Kirala, R.S., and Swayer, R.,Pearson’s Chemical analysis of foods, Churchil Living Stone, Edinburough (1981).
- Krishnakumar, G., Krishnankutty, K., Mohanan, S., Raju, K., and Panicker, C.Y.,Mat. Sci. Res. India, 4(2): 465 (2007).
CrossRef
- Krishnakumar, G., Krishnankutty, K., Raju,K., Mohanan, S., Varghese, H.T., and Panicker, C.Y., Mat. Sci. Res. India, 4(2): 481 (2007).
CrossRef
- Mohanan, S.,Vaidyan, V.K., and Kurian, K.V.,Appl. Phys. Lett., 70: 805 (1997).
CrossRef
- Marcus, Y., Introduction to liquid state Chemistry, John Wiley and Sons ,New York (1997).
- Kagan, J., Organic Photo chemistry, Principles and applications, Academic Press, London (1993).
- Thirumaran, R., and Sabu, J., Indian J. Pure Appl.Phys., 47: 87 (2009).
- Thiyagarajan, R., and Palaniappan, L., Indian J. Pure Appl. Phys., 46: 852 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal